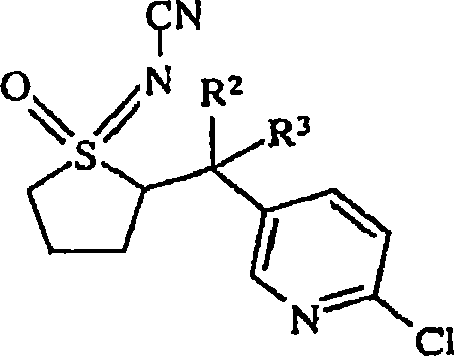
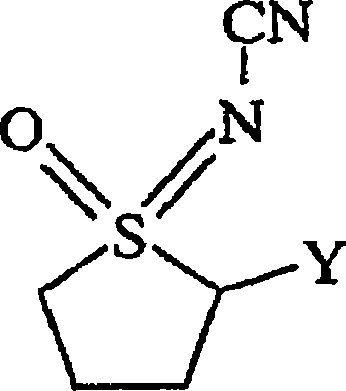
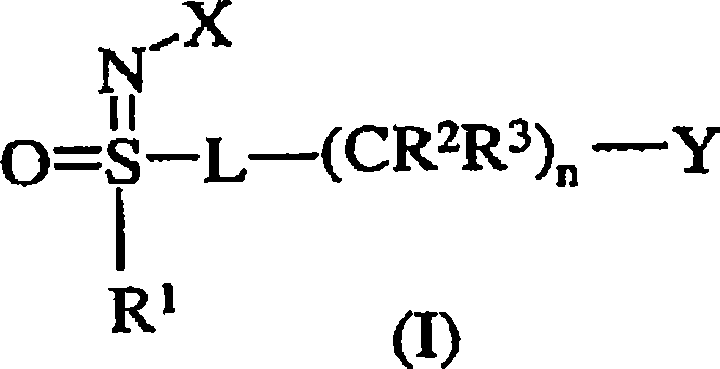Use of n-substituted sulfoximines for control of invertebrate pests
An invertebrate, Chinese-style technology with applications, chemicals for biological control, biocides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I-III
[0035] Examples I-III. Preparation of N-substituted sulfoximes
[0036] Sulfoximes I-III were prepared by methods previously disclosed in US Patent Publication No. 2005 / 0228027.
Embodiment I
[0037] Example I. [(6-Chloropyridin-3-yl)methyl](methyl)oxidation-λ 4 -Preparation of sulfoiminonitrile (1)
[0038]
[0039] A solution of 5-chloromethyl-2-chloropyridine (8.1 g, 50 mmol) in ethanol (50 mL) was added to a stirred suspension of solid sodium methylthiolate (4.2 g, 60 mmol) in 100 mL of ethanol. An exothermic reaction was observed during the addition and the mixture was stirred overnight at room temperature.
[0040] The solvent ethanol was removed under reduced pressure, and the residue was redissolved in ether-ethyl acetate solvent and mixed with brine. The two phases were separated, and the organic layer was washed with anhydrous Na 2 SO 4 Drying, filtration, concentration and purification by flashing through a plug of silica gel eluting with 40% EtOAc(ethyl acetate) / hexanes gave 8.14 g of 2-chloro-5-[(methylthio)methyl]pyridine , which was a colorless oil with a yield of 94%. The product was analytically pure and used directly in the next react...
Embodiment II
[0048] Example II. [1-(6-Chloropyridin-3-yl)ethyl](methyl)oxidation-λ 4 -Preparation of sulfoiminonitrile (2)
[0049]
[0050] To a solution of N-cyanosulfoxime (1) (0.34 g, 1.5 mmol) and hexamethylphosphoric triamide (HMPA) (0.14 mL, 0.8 mmol) in 15 mL of anhydrous tetrahydrofuran (THF) at -78°C A solution of 0.5M potassium bis(trimethylsilyl)amide (KHMDS) in toluene (3.6 mL, 1.8 mmol) was added dropwise. After 45 minutes, iodomethane (0.11 mL, 1.8 mmol) was added via syringe in one portion. After 10 minutes, the temperature was raised to 0°C. After stirring for 1.5 h, the reaction mixture was washed with saturated NH 4 Quenched with aqueous Cl solution, diluted with brine, and washed with CH 2 Cl 2 Extract three times. The combined organic layers were washed with Na 2 SO 4 Dry, filter and concentrate. The residue was purified twice on silica gel, first with 2% MeOH / CH 2 Cl 2 (v / v) eluted second with 9% acetone / CH 2 Cl 2 (v / v) was eluted to obtain 0.217...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com