Preparation method of 5'-deoxidization-2',3'-diacetyl-5-fluoro-cytidine
A technology of flucytidine and diacetyl, applied in the field of 5'-deoxy-2', can solve the problem of high cost of anti-tumor drugs, achieve the effects of increased synthesis yield, accelerated reaction speed, and reduced manufacturing cost and price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
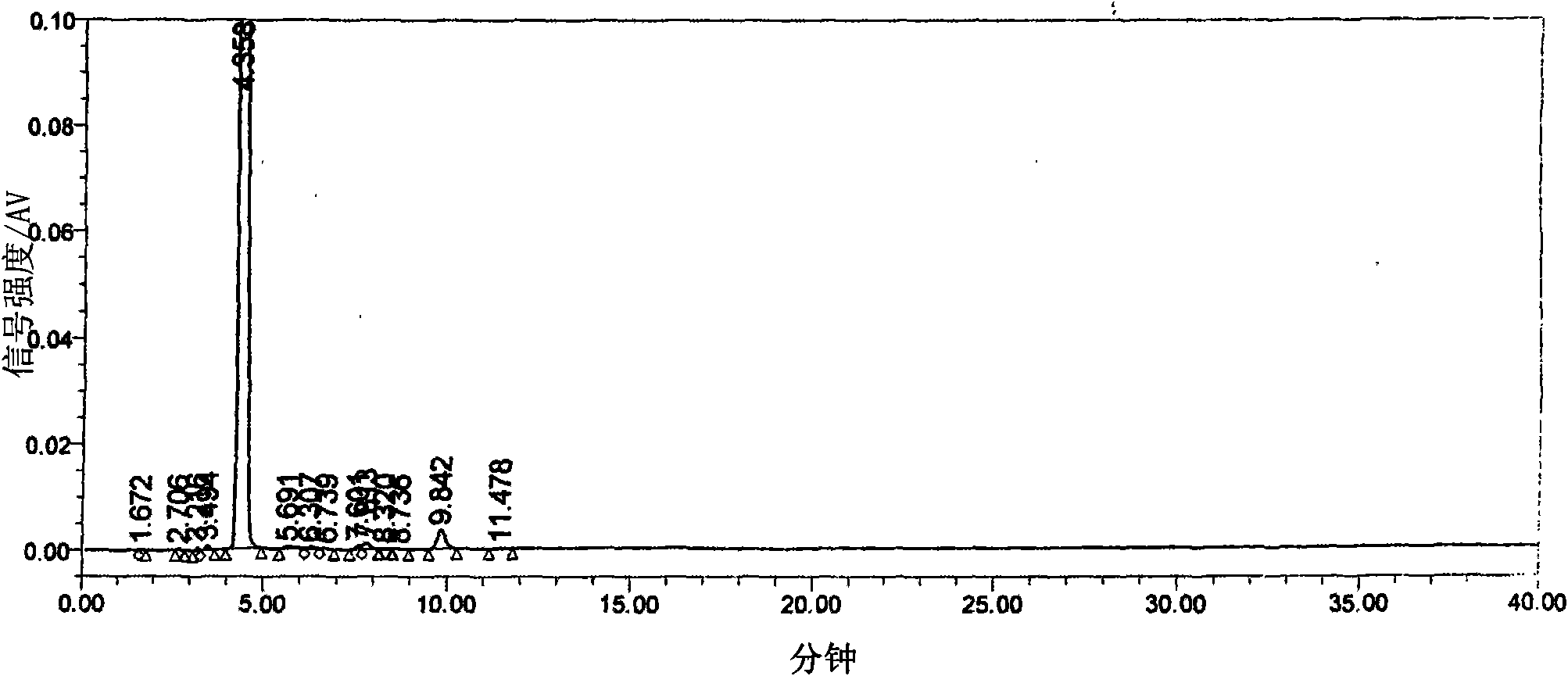
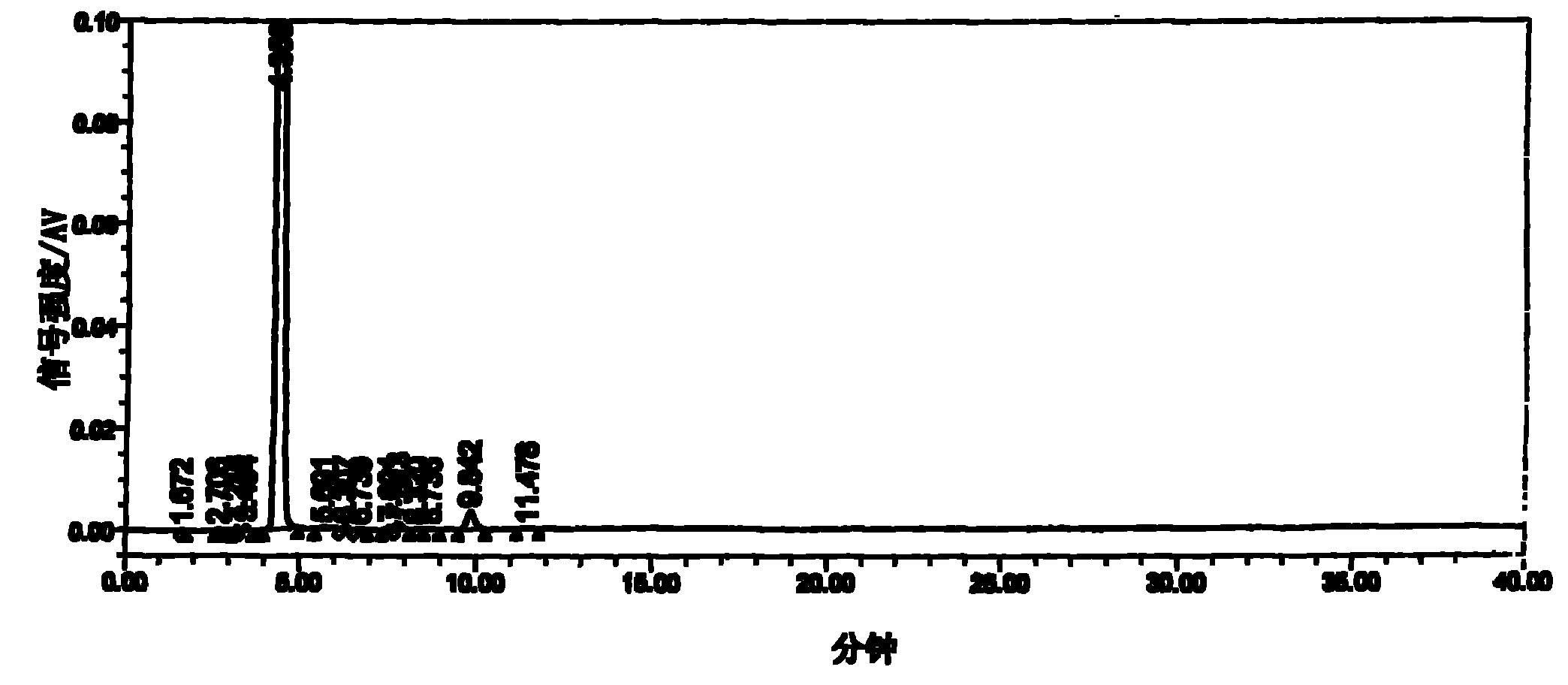
Image
Examples
Embodiment 1
[0020] The three-necked flask equipped with a stepless speed-regulating stirrer, a thermometer of 0°C-150°C, and a reflux and can be turned into a distillation condenser is installed in an automatic constant temperature electric heating oil bath. Weigh 12.9g (0.10mol) of 5-fluorocytosine, put it into a three-neck flask, then put in 100ml of toluene, 22ml of hexamethyldisilazane, and 0.25g of ammonium sulfate, stir at high speed, raise the temperature above 100°C, and reflux for 3.5 After 1 hour, the toluene was distilled off under reduced pressure until the feed liquid was viscous, and a mixture of 24.5 g (0.094 mol) of 5-deoxy-1,2,3-triacetyl ribose and 120 ml of dichloromethane was added. Replace the oil bath with the low-temperature bath liquid of the low-temperature cooling circulation pump, cool the feed liquid to 0°C, add a mixed solution of 13ml of anhydrous tin tetrachloride and 30ml of dichloromethane, keep warm and stir at a slow speed for 3 hours. The reaction solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com