1H-quinolin-4-one compounds, with affinity for the GABA receptor, processes, uses and compositions
A compound, quinoline technology, applied in the direction of drug combination, active ingredient of heterocyclic compounds, organic chemistry, etc., can solve problems such as the central nervous system of undisclosed related compounds
Inactive Publication Date: 2009-09-02
FERRER INT SA
View PDF2 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
No related compounds belonging to this chemical class have been disclosed as having GABA A Receptor ligand activity and any other central nervous system activity
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
preparation example Construction
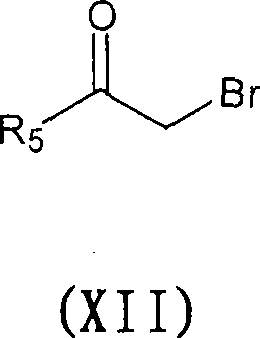
[0273] Compounds 1-146, Table 4 were prepared according to the above-mentioned Preparation Examples.
[0274] Table 4
[0275]
[0276]
[0277]
[0278]
[0279]
[0280]
[0281]
[0282]
[0283]
Embodiment 1
[0284] Composition example 1: 5 mg tablet
[0285]
Embodiment 2
[0286] Composition Example 2: 10 mg Capsules
[0287]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to view more PUM
 Login to view more
Login to view more Abstract
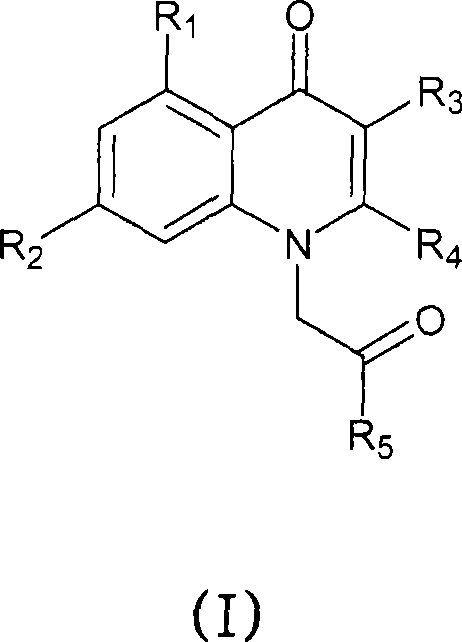
The invention provides new 1H-quinolin-4-one compounds of formula (I), wherein R1, R2, R3, R4 and R 5 have different meanings, and pharmaceutically acceptable salts and hydrates thereof. Compounds of formula (I) are useful for treating or preventing diseases associated with GABAA receptors modulation, anxiety, epilepsy, sleep disorders including insomnia, and for inducing sedation-hypnosis, anesthesia, sleep and muscle relaxation. The invention also provides synthetic procedures for preparing said compounds.
Description
technical field [0001] The present invention relates to having GABA A A new class of agents for receptor affinity. In particular, the present invention relates to novel 1H-quinolin-4-one compounds useful for the treatment or prevention of anxiety, epilepsy and sleep disorders including insomnia, and for inducing sedation-hypnosis, anesthesia, sleep and muscle relaxation. Background technique [0002] GABA A receptor (gamma-aminobutyric acid A ) are pentameric proteins that form membrane ion channels. GABA A The receptors have been implicated in the regulation of sedation, anxiety, muscle tone, epileptogenic activity and memory function. These effects should be attributed to the identified GABA A Subunits of receptors, especially alpha 1 -subunit and alpha 2 - Subunits. [0003] Sedative effect via alpha 1 - Subunits are regulated. Zolpidem is characterized by the presence of alpha 1 - The receptors have high affinity and their sedative and hypnotic effects are m...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to view more Application Information
Patent Timeline
 Login to view more
Login to view more Patent Type & Authority Applications(China)
IPC IPC(8): C07D215/06C07D215/18C07D401/06C07D401/14C07D409/06C07D413/06C07D405/06C07D417/06A61K31/47A61P25/20A61P25/22
CPCC07D401/14C07D405/06C07D417/06C07D215/18C07D401/06C07D215/06C07D409/06C07D413/06A61P21/02A61P25/00A61P25/08A61P25/20A61P25/22
Inventor J·L·法尔科A·帕洛莫A·古格列塔
Owner FERRER INT SA
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap