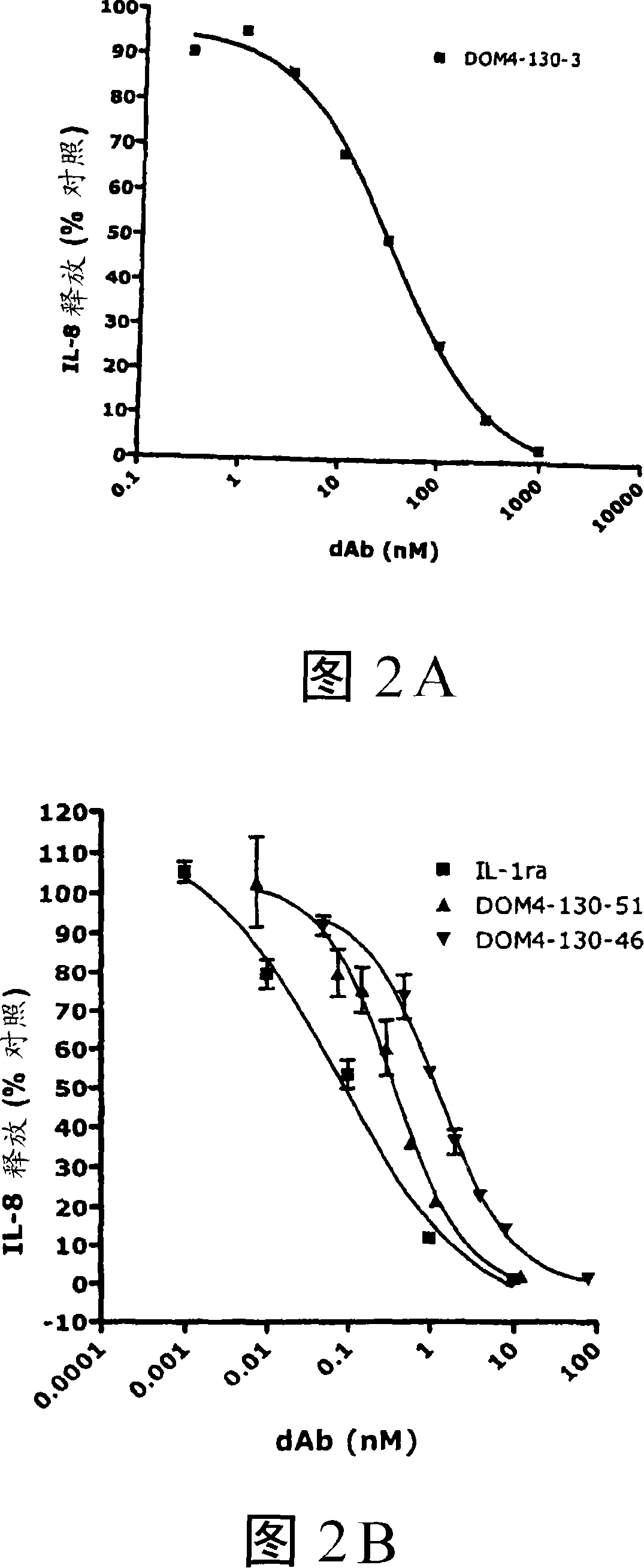
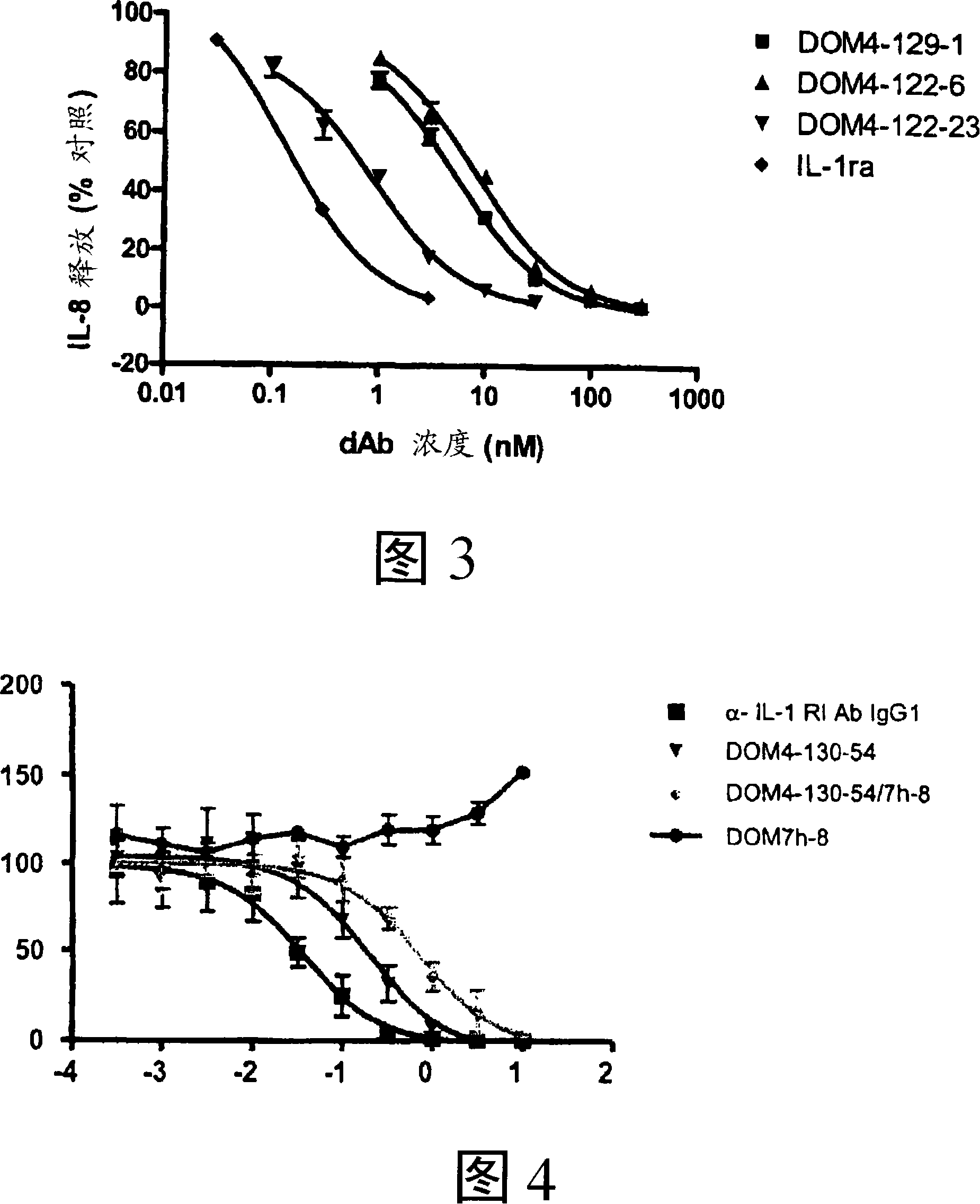
Anti-il-1r1 single domain antibodies and therapeutic uses
An interleukin and antagonist technology, which can be used in respiratory diseases, receptors/cell surface antigens/cell surface determinants, allergic diseases, etc., and can solve problems such as failure to meet primary endpoints
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0246] Example 1. IL-1R1 Immunoglobulin Variable Domain Antagonists
[0247] method
[0248] selection and filtering
[0249] For primary selection, the 4G-K2 library of VK dAbs was panned against the IR1-Fc fusion protein (Axxora, Nottingham, UK). Domain antibodies selected from the primary screen were subjected to three further rounds of screening. The first round was carried out with protein G coated with magnetic beads (Dynal, Norway); the second round was carried out with anti-human IgG beads and 10 nMIL-1R1-Fc (Novagen, Merck Biosciences, Nottingham, UK); the third round was carried out with Protein G beads and 1 nM IL-1R1-Fc were performed. (See Henderikx et al, Selection of antibodies against biotinylated antigens. Antibody Phage Display: Methods and protocols, Ed. O'Brien and Atkin, Humana Press (2002).) Elution was performed at each stage with 1 mg / ml trypsin-PBS . For the affinity maturation screen, the method described above was used with the following modif...
Embodiment 2
[0275] Example 2. IL-1R1 Antagonist Subchronicity of COPD in C57BL / 6 Mice valid within the model
[0276] In this study, IL-1R1 antagonists (and half-life-extending fusion proteins including IL-1ra and binding to mouse serum albumin) were administered alone or in combination with TNFR1 at intervals starting 24 hours before initial tobacco smoke exposure. 48 hours intraperitoneal injection. The effects of tobacco smoke-induced changes in indices of lung inflammation induced by 11 consecutive days of tobacco smoke exposure were examined 24 hours after the final exposure. This result demonstrates that IL-1R1 antagonists are effective in the mouse model. ENBREL(R) (etanercept; Immunex Corporation), which binds TNF and thus antagonizes TNFR1, was used as a comparator.
[0277] Test compound 1: ENBREL(R) (etanercept; Immunex Corporation)
[0278] Test compound 2: IL-1ra / anti-S A dAb (IL-1ra fused to DOM7m16)
[0279] Test compound 3: a mixture of PEG DOMIm (anti-TNFR1 dAb in...
Embodiment 3
[0299] Example 3. Local Administration of Immunoglobulin Variable Domains to Lung Tissue
[0300] In this study, a domain antibody that binds egg lysozyme (V H ) for topical administration to lung tissue by intranasal administration. This result demonstrates that domain antibodies can be delivered locally to a lung tissue model.
[0301] method
[0302] Female mice (C57BL / 6) were all bred and undocumented specific organisms (16-20 g) (Charles River) were bred and housed in groups of 5 in ventilated, solid bottomed, poplar sawdust beds (IVC). The environment (airflow, temperature and humidity) inside the cages was controlled by an IV system (Techniplast). The domain antibody HEL4 binds the V of egg lysozyme H . (See, Jespers et al. J. MoI. Biol., 337:893-903 (2004). The HEL-4 monomer containing the HA-tag for detection was diluted with 20 mM sodium citrate and 100 mM NaCl at pH=6.0. Mice were lightly anesthetized (isofluorane / O 2 ) and slowly drop 50 microliters of dAb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com