Compositions and methods for treatment of neovascular diseases
A technology of neovascularization and composition, which is applied in the field of treatment of neovascular diseases and can solve problems such as increased effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
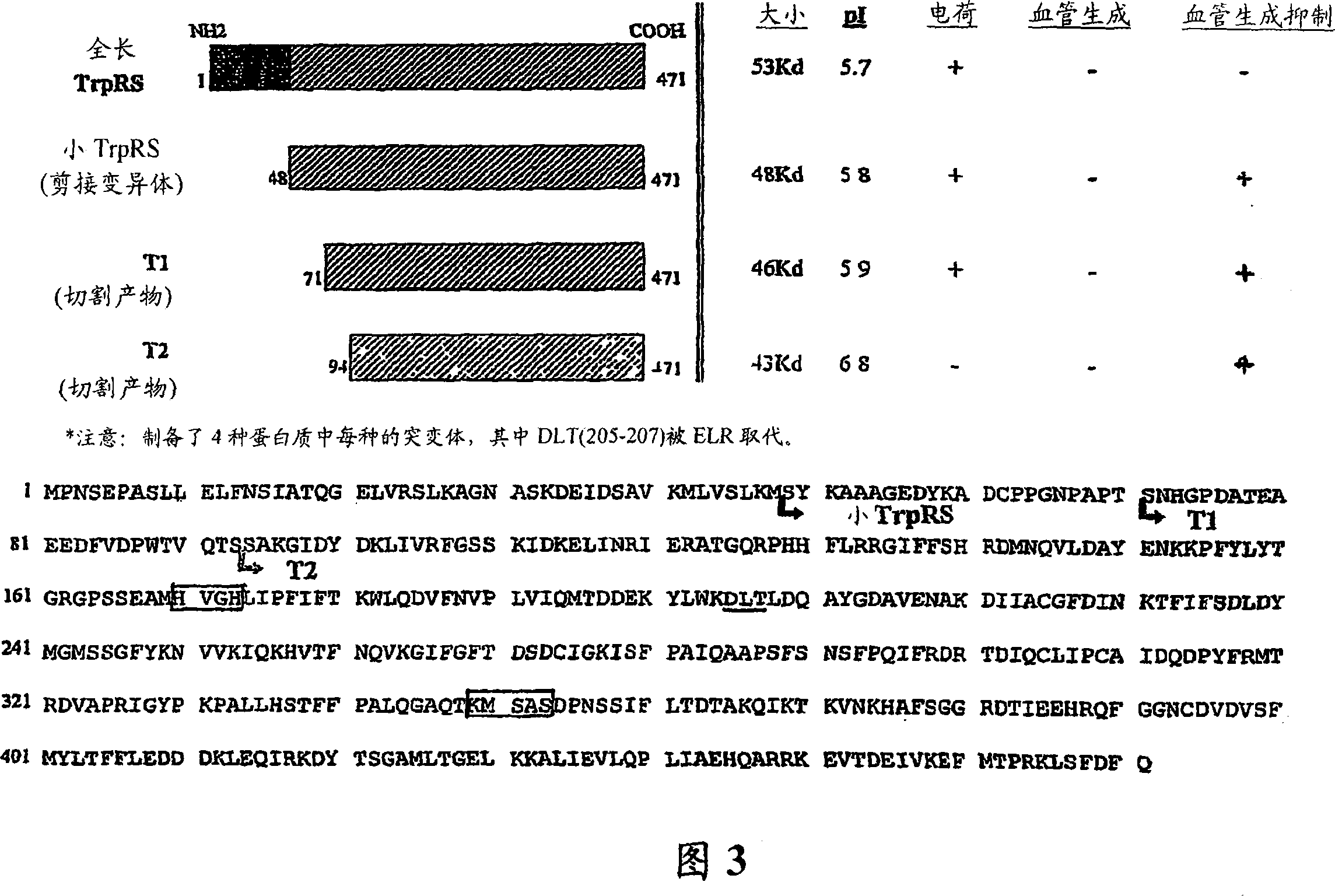
Image
Examples
Embodiment 1
[0125] Example 1. Treatment of neonatal mouse eyes with a combination of peptide mimetic integrin signaling inhibitors and VEGF signaling inhibitors
[0126] Following the general procedure for the angiogenesis assay described above ("General Procedure"), on postnatal day 8 (P8), neonatal Balb / C mouse eyes were injected intravitreal at a concentration of 0.25x the integrin signaling inhibitor compound (1 ) (5 mice), VEGF aptamer compound (2) at a concentration of 0.5x (5 mice), or a combination of compound (1) at a concentration of 0.25x and compound (2) at a concentration of 0.5x (6 mice ). As a control, another group of 6 mice received intravitreal injection of PBS only. Following the general protocol, mice were euthanized at P12 and retinas were removed from injected eyes, stained, mounted and evaluated microscopically. Vascularity in the second (external retinal vessels) layer was assessed based on the percentage of vascularization compared to control eyes. The results ...
Embodiment 2
[0128] Example 2. Treatment of Neonatal Mouse Eyes with Combinations of TrpRS Angiogenesis Inhibitory Fragments and VEGF Signaling Inhibitors
[0129] According to the general method, at P4, neonatal Balb / C mouse eyes were intravitreally injected with 0.1x concentration of T2-TrpRS (8 mice), 0.1x concentration of VEGF aptamer compound (2) (8 mice) or Combination of T2-TrpRS at 0.1x concentration and Compound (2) at 0.1x concentration (10 mice). As a control, another group of 8 mice received only intravitreal injection of PBS. Following the general protocol, mice were euthanized at P12 and retinas were removed from injected eyes, stained, mounted and evaluated microscopically. Vascularity in the second (external retinal vessels) layer was assessed based on the percentage of vascularization compared to control eyes. The results are shown in Table 2 and Fig. 6, Fig. 7, Fig. 8 and Fig. 9.
[0130] % suppression:
Embodiment 3
[0131] Example 3. Treatment of Neonatal Mouse Eyes with Combinations of TrpRS Angiogenesis Inhibitory Fragments and VEGF Signaling Inhibitors
[0132] According to the general method, at P4, the eyes of neonatal Balb / C mice were intravitreally injected with 1x concentration of T2-TrpRS (8 mice), 1x concentration of VEGF aptamer compound (2) (8 mice) or 1x concentration Combination of T2-TrpRS and compound (2) at 1x concentration (10 mice). As a control, another group of 6 mice received intravitreal injection of PBS only. Following the general protocol, mice were euthanized at P12 and retinas were removed from injected eyes, stained, mounted and evaluated microscopically. Vascularity in the second (external retinal vessels) layer was assessed based on the percentage of vascularization compared to control eyes. The results are shown in Table 3 and Figure 10, Figure 11, Figure 12 and Figure 13.
[0133] % suppression:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com