Cornel extract and use thereof
A technology of Cornus officinalis and its extracts, which is applied in the field of preparing medicines for treating pain, its active ingredients, autoimmune diseases or inflammations, Cornus officinalis extracts, and can solve the problems of undisclosed components and proportions of Cornus officinalis extracts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
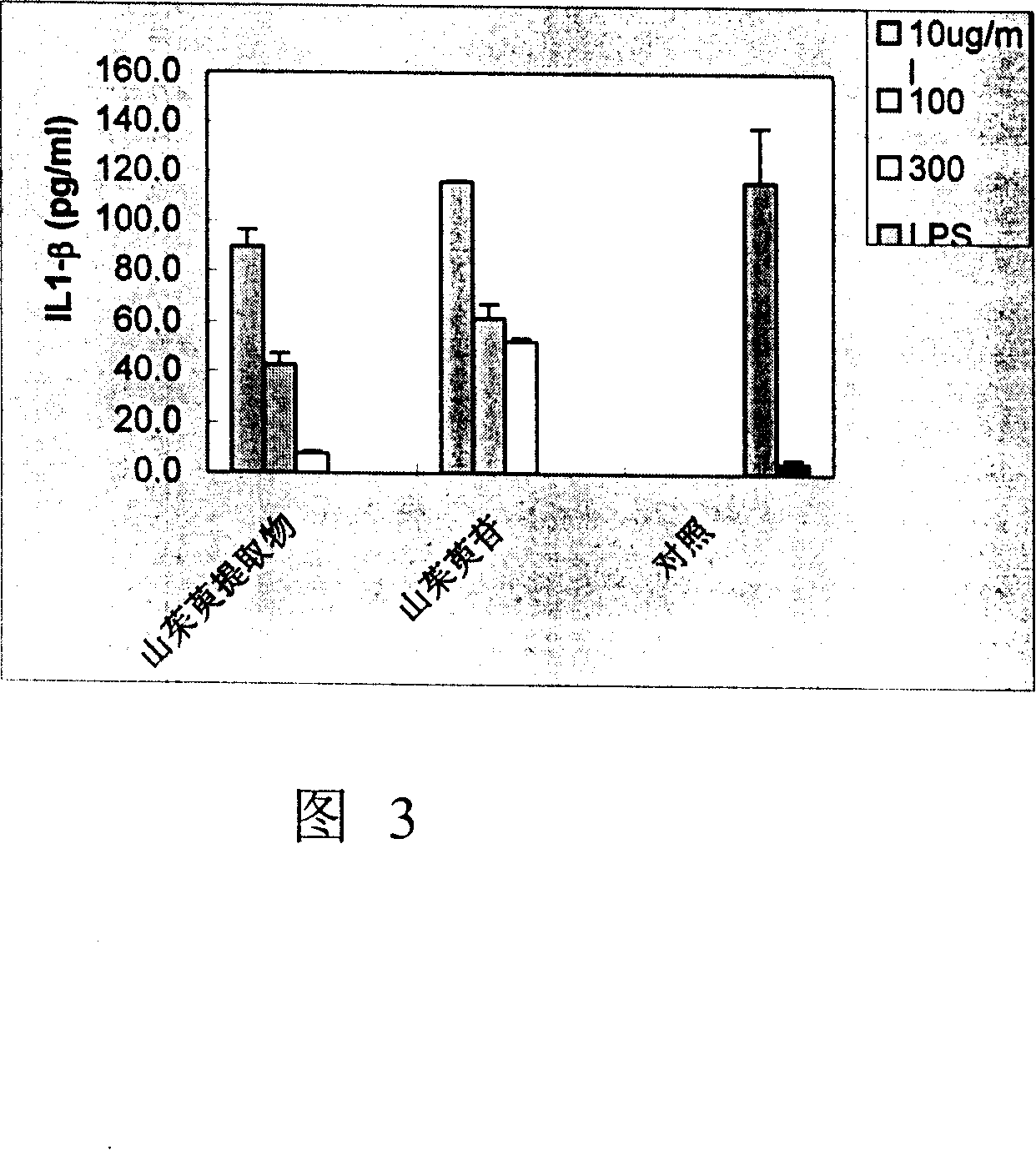
Examples
Embodiment 1
[0053] Example 1 Preparation of Cornus officinalis Extract and Determination of Polyphenol Content Therein by Skin Powder Method
[0054] Cornus officinalis 500g, add water 5000mL, decoct 3 times, the degree of decoction is slightly boiled, the decoction time is 2 hours each time, the decoction liquid is filtered, combined, and set aside for later use; The ratio of 1:2 is packed into the column, and then the above-mentioned water decoction is passed through the macroporous adsorption resin column at a flow rate of 20 ml / min. After passing through completely, the resin column is eluted with water, 10% ethanol and 95% ethanol . The eluate was collected and vacuum-dried to obtain 48 g of Cornus officinalis extract.
[0055] Take 2.0076g (W) of the above-mentioned Cornus officinalis extract, dissolve it in 500mL of water, take 25mL of the solution, concentrate it to dryness, and then dry it at 105°C for 3 hours to obtain a solid product, the weight of which is T 1 = 0.0966g.
...
Embodiment 2
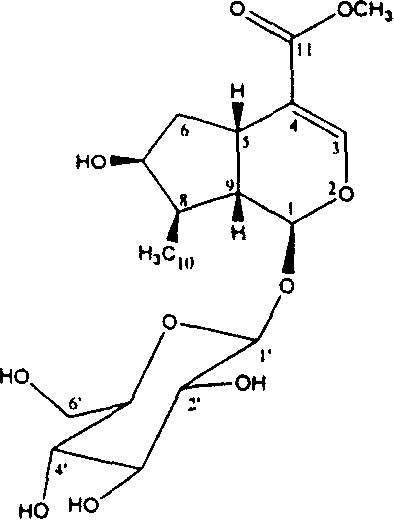
[0059] Example 2 Separation and Identification of Three Active Components of Cornus officinalis
[0060] Isolation and identification of loganin:
[0061] 100g of Cornus officinalis can be soaked three times in 500mL of 95% ethanol, and the extracts can be combined to obtain 10g of concentrate. The concentrate was subjected to vacuum column chromatography and eluted with petroleum ether, chloroform and methanol in sequence. After concentration of methanol eluent, silica gel column chromatography (eluent CH 2 CL 2 / CH 3 OH=6:4), to get loganin 875mg, its Rf value=0.57 (developing agent: 0.1M KH 2 PO 4 : 0.1MH 3 PO 4 :CH 3 CN=7:7:4). Its spectral data are:
[0062] [α] D 25 =-81°(c 0.1, H 2 O), UV(λ max , nm) 238 (4.0); IR: (cm -1 , KBr): 3350, 3300, 1715, 1650, 1440, 1300, 1080;
[0063] 1 H NMR (D 2 O, 300MHz) δppm: 7.42 (s, H-3), 5.39 (d, J=3.6Hz, H-1), 4.14 (m, H-7), 3.73 (s, OCH3), 3.05 (m, H -5), 2.16(m, H-6a), 2.10(m, H-9), 1.88(m, H-8), 1.73(m, H-6b), ...
Embodiment 3
[0075] Example 3 HPLC chromatogram and quantitative analysis of Cornus officinalis extract
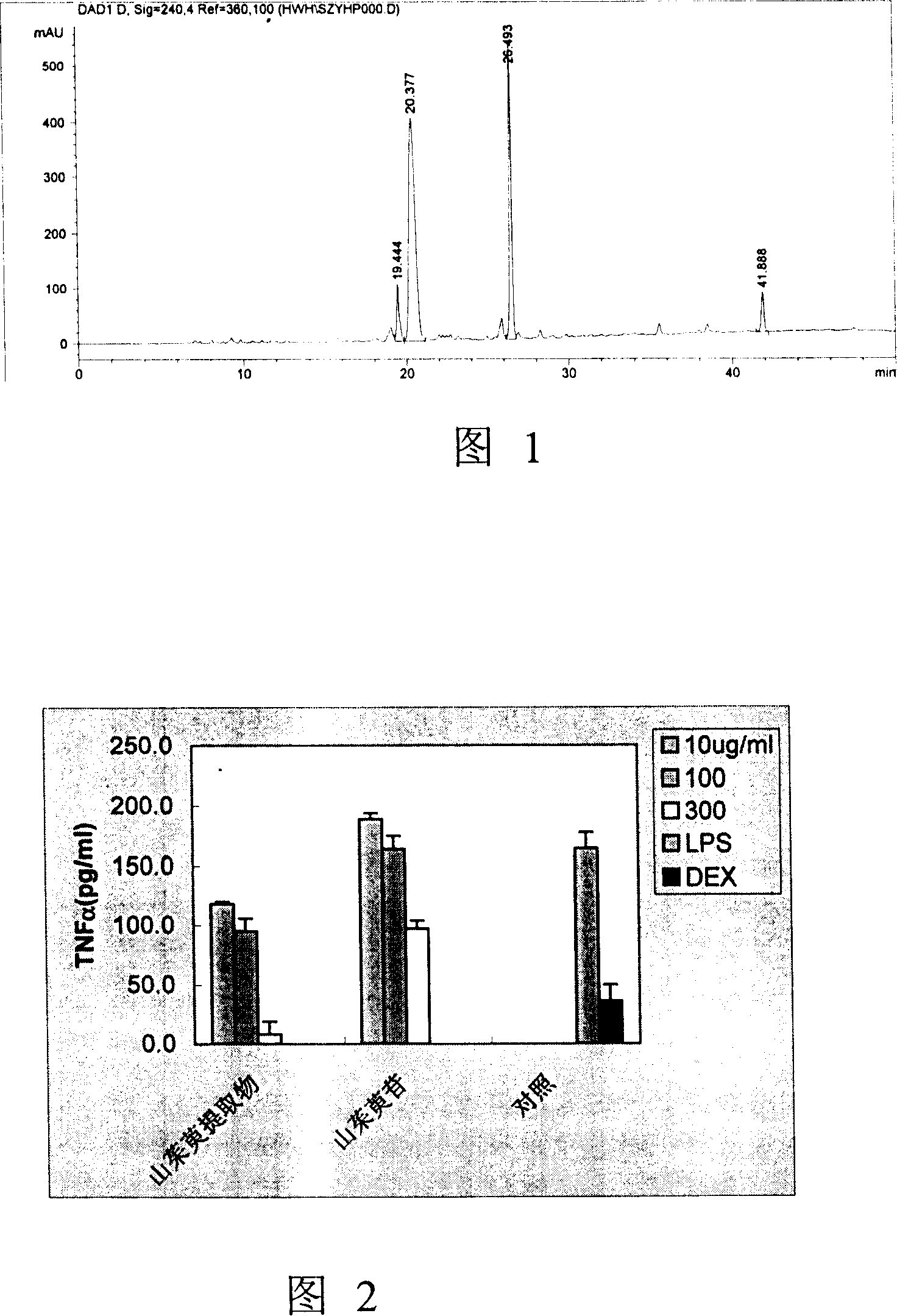
[0076]Quantitative analysis of Cornus officinalis extract (Example 1) was carried out by HPLC system. Instrument: Agilent 1100, the set wavelength is 240nm, the analytical column is Zorbax SC184.6*150mm, and the flow rate is 1ml / min. The mobile phase is CH 3 CN and 0.1% H 3 PO 4 solution. The gradient status is: CH 3 CN increments from 0% to 25% in 50 minutes. During the next 5 minutes, CH 3 CN is incremented to 100%. Obtain the HPLC collection of illustrative plates shown in accompanying drawing 1.
[0077] The reference substances of loganin, morroniside, and corniside were all isolated and purified in this laboratory. Through HPLC analysis, the purity is above 96%.
[0078] The Cornus officinalis extract of the present invention (Example 1) contains 9.7% loganin (retention time 20.4 minutes), morroniside 17.5% (retention time 26.5 minutes), and corniside 2.6% (retention ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com