Methods of sustaining dietary ketosis and its effects on lipid profile
a ketogenic and lipid profile technology, applied in the field of ketogenic supplements, can solve the problems of difficult for most humans, physiological decrease of glucose and insulin, etc., and achieve the effects of suppressing hunger, rapid and sustained elevation of blood ketones, and improving metabolic health and performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
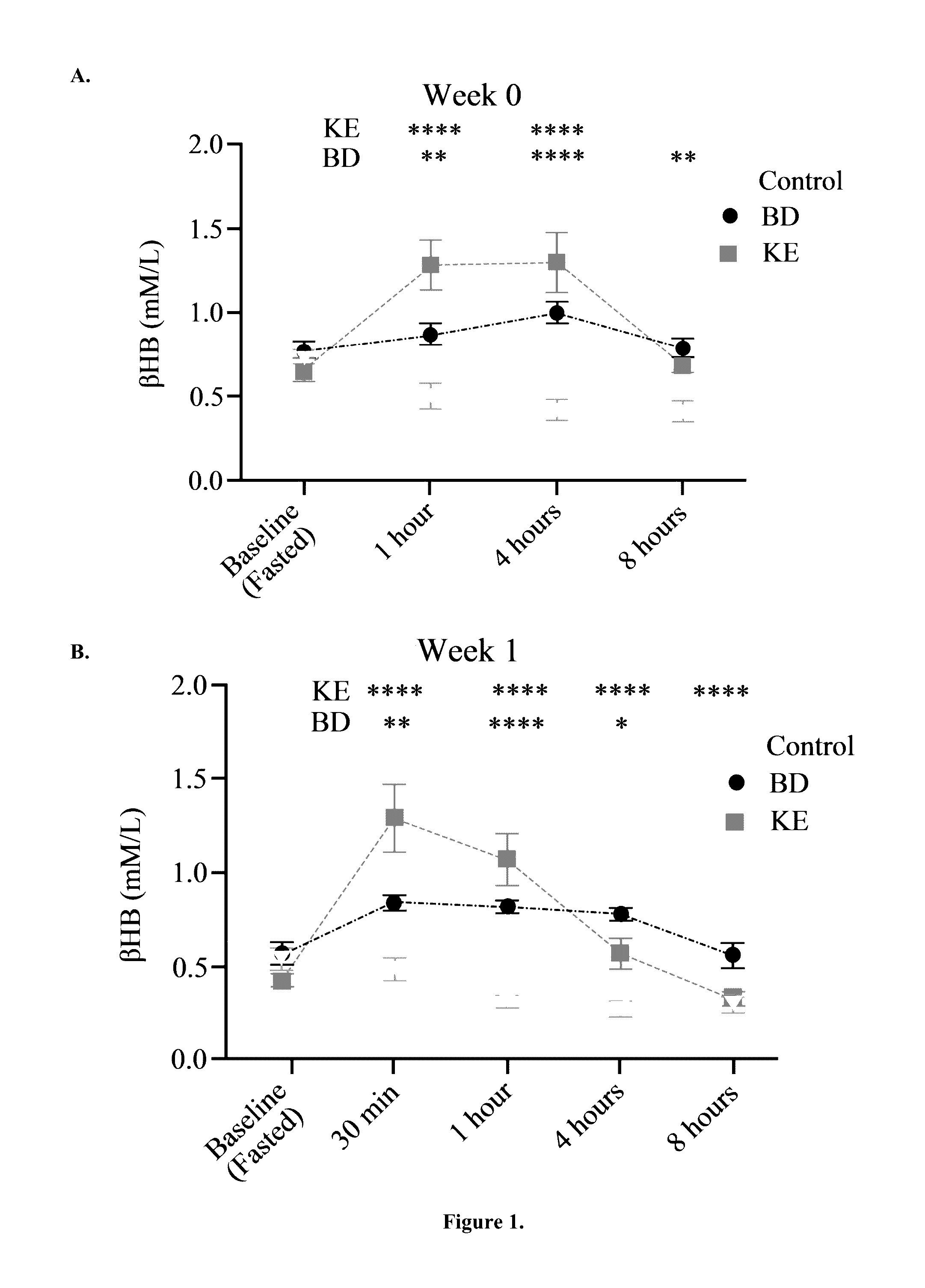
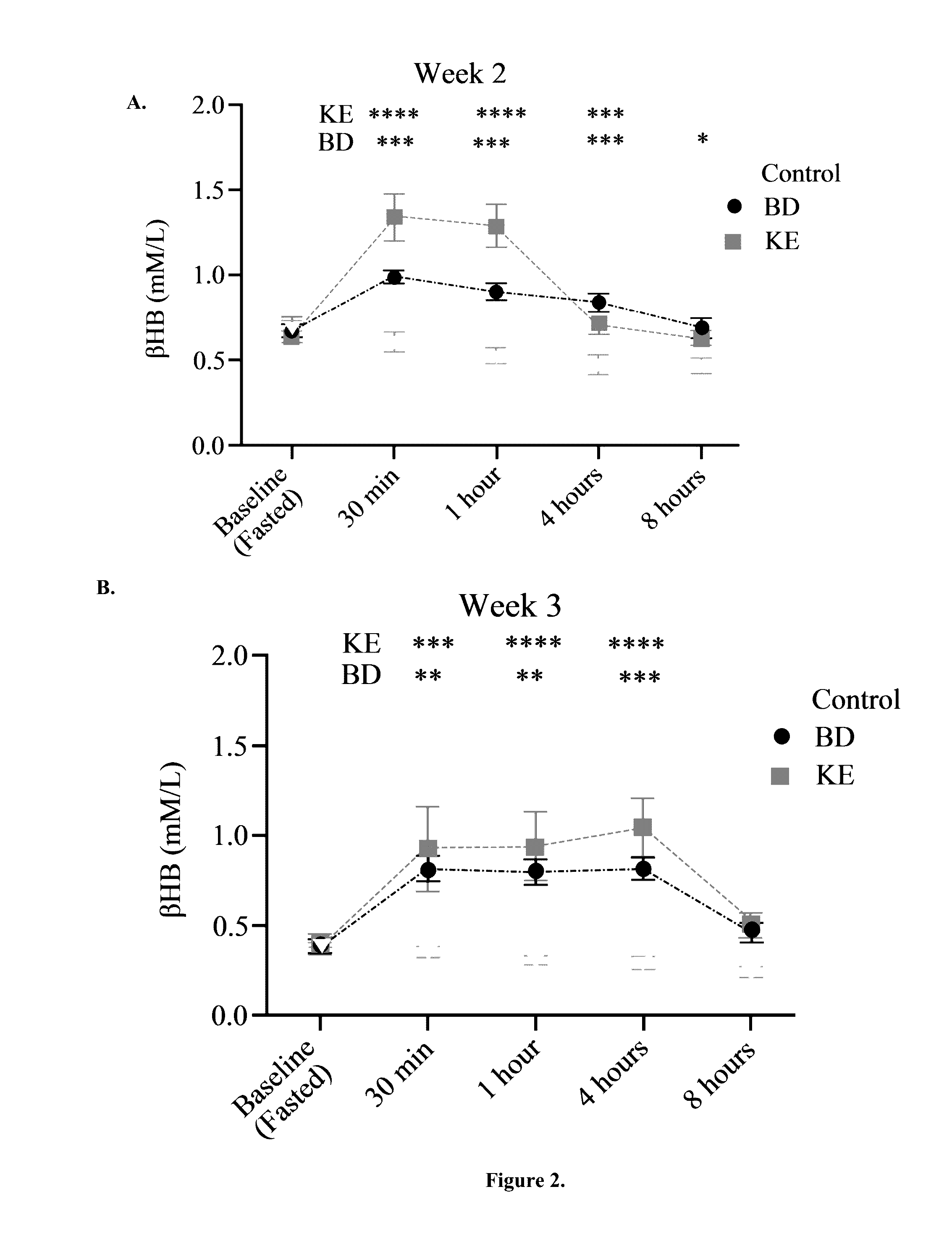
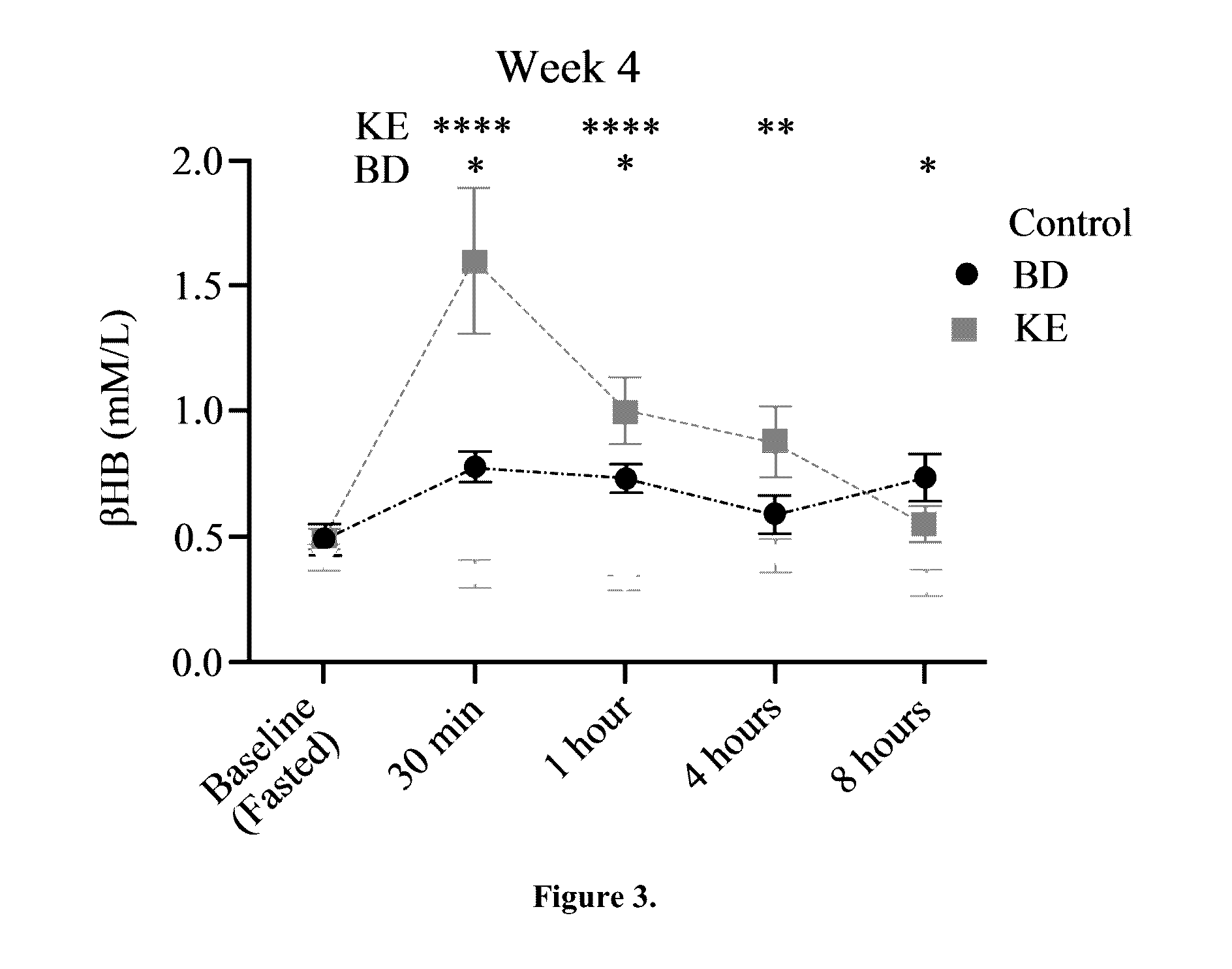
[0052]BD was purchased from Sigma (Milwaukee, Wis.). R,S-1,3-butanediol acetoacetate diester (KE) synthesized as previously described (D'Agostino, et al., Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am J Physiol Regul Integr Comp Physiol. 2013 May 15; 304(10):R829-36). Briefly, R,S-1,3-butanediol and t-butylacetoacetate were purchased from Sigma (Milwaukee, Wis., USA). All commercial solvents and reagents used were high-purity reagent-grade materials. KEs were synthesized by transesterification of t-butylacetoacetate with R,S-1,3-butanediol (Savind Inc., Seymour, Ill.). The resultant product consisted of a mixture of monoesters and diester, the ratio of which could be adjusted by varying the stoichiometry of reactants. Following synthesis the crude product was distilled under reduced pressure to remove all solvents and starting materials, and the resultant BD-AcAc or BD-AcAc2 was obtained and assessed for purity using gas chr...
example 2
[0061]Animals treated from Example 1 were sacrificed by CO2 between 4-8 hours after gavage-treatment at the end of 4 weeks (28 days at the dose of 5-10 g / kg), which were determined to be peak ketone elevation. Brain, Lungs, Liver, Kidneys, Spleen and Heart were harvest and weighed using AWS-1000 1 kg portable digital scale (AWS, Charleston, S.C.). Organs were then either flash frozen in liquid nitrogen or preserved in paraformaldehyde for future analysis.
[0062]KE ketone supplements significantly decreased the weight of the liver in the rats. BD supplemented animals showed no significant change in non-liver organ weight, as seen in FIG. 14(A). However, KE treatment did result in a statistically significant reduction in liver weight, as seen in FIG. 14(B).
[0063]Livers from rats administered maximum tolerable dose of ketone supplementation were assessed via histology. The livers from rats treated 4-weeks with ketone supplementation appeared normal in color, size and texture in all grou...
example 3
[0065]Ketones, beta-hydroxybutyrate (βHB) and acetoacetate (AcAc), are derived from acetyl-CoA generated from the oxidation of fatty acids in the liver. In extrahepatic tissues, they are converted back to acetyl-CoA where they serve as important fuel sources—especially in the heart and skeletal muscle. Most tissues in mature animals, under nutrient replete conditions, do not use ketones but can adapt to their use during starvation or prolonged exercise when glycogen stores become depleted. The underlying hypothesis behind the use of ketogenic agents for induction of nutritional ketosis is that ketones are a metabolic substrate that can be exploited through the oral administration of specific forms of supplementation. This global metabolic profiling study was conducted to examine the systemic and brain metabolic responses of healthy rats subjected to two different diets capable of inducing nutritional ketosis.
[0066]Rats were treated as described in Example 1, and brain and serum samp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy | aaaaa | aaaaa |
| physical performance | aaaaa | aaaaa |
| resilience | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com