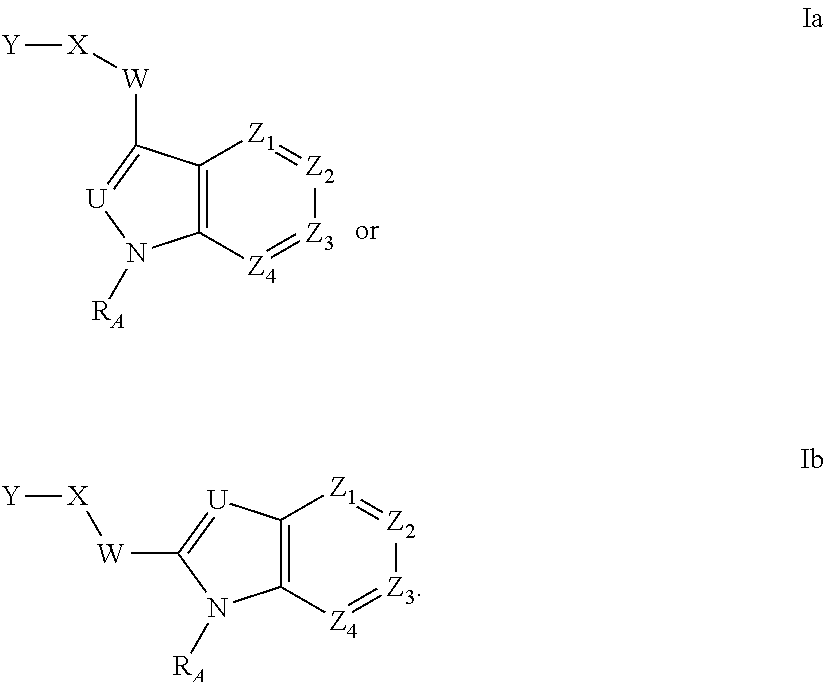
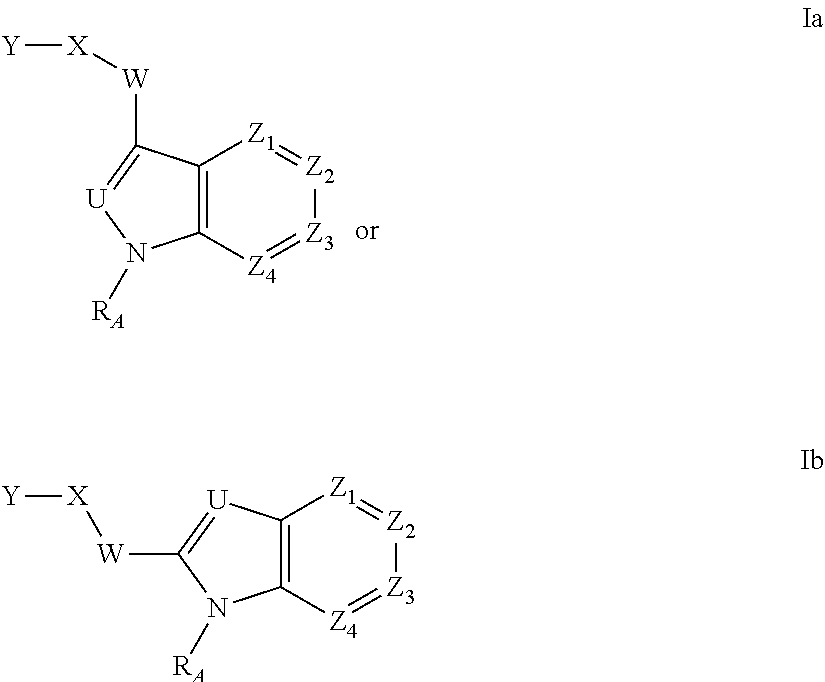
Heteroaryl Amide Analogues
a technology of heteroaryl amide and analogues, which is applied in the field of heteroaryl amide analogues, can solve the problems of acute or chronic pain, more debilitating, and opiates, such as morphine, are potent analgesics, and achieve the effect of inhibiting the death of retinal ganglion cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
N-(Adamantan-1-ylmethyl)-1-pyrimidin-2-yl-1H-indole-3-carboxamide
Step 1. Methyl 1-(pyrimidin-2-yl)-1H-indole-3-carboxylate
[0261]
[0262]Potassium t-butoxide (2.52 g, 0.022 mol) is added to a mixture of methyl 1H-indole-3-carboxylate (3.5 g, 0.02 mol) and 2-chloropyrimidine (2.28 g, 0.02 mol) in 50 mL of dioxane. The reaction mixture is heated to 110° C. and stirred for 20 h. The dioxane is removed in vacuo, and the residue is diluted with water (100 mL). The solid is filtered and purified by silica gel column chromatography (15% EtOAc / DCM) to afford the title compound as a white solid.
Step 2. 1-(Pyrimidin-2-yl)-1H-indole-3-carboxylic acid
[0263]
[0264]1.0 N aqueous NaOH (10 mL) is added to a mixture of methyl 1-(pyrimidin-2-yl)-1H-indole-3-carboxylate (1.4 g, 0.0055 moles) in 50 mL of EtOH and heated at 70° C. for 4 h. The reaction mixture is concentrated in vacuo, diluted with water (50 mL), acidified with concentrated HCl to pH 2.0. The white solid separated is filtered, washed with w...
example 2
N-[(1-Pyridin-3-ylcyclohexyl)methyl]-1-pyrimidin-2-yl-4-(trifluoromethyl)-1H-indole-3-carboxamide
Step 1. 4-Bromo-1H-indole-3-carbaldehyde
[0267]
[0268]A round bottom flask charged with 50 mL of DMF is cooled to 0° C., and phosphorous oxychloride (8.1 mL, 88 mmol) is added dropwise. After stirring for approx. 5 min, a solution of 4-bromoindole (5.0 mL, 40 mmol) in 50 mL of DMF is added dropwise. The ice bath is removed, and the reaction mixture is stirred for 1 h at rt. The reaction becomes a very thick suspension. The mixture is cooled back to 0° C. and carefully quenched with 22g of KOH in 80 mL of water. The resulting mixture is partitioned between EtOAc (200 mL) and sat. NaHCO3 (100 mL). The EtOAc layer is washed with brine (100 mL) and water (100 mL), dried (Na2SO4), filtered, and evaporated in vacuo to give a brown solid, which is triturated with ether to afford the title compound as an off-white solid.
Step 2. 4-(Trifluoromethyl)-1H-indole-3-carbaldehyde
[0269]
[0270]Methyl 2-(fluo...
example 3
4-Chloro-N-[(1-pyridin-3-ylcyclohexyl)methyl]-1-pyrimidin-2-yl-1H-indole-3-carboxamide
Step 1. 1-(4-Chloro-1H-indol-3-yl)-2,2,2-trifluoroethanone
[0280]
[0281]Trifluoroacetic anhydride (27.5 mL, 200 mmol) is added to a solution of 4-chloroindole (25.0 g, 165 mmol) and DMF (170 mL) under N2 over 30 min. The reaction vessel is sealed. After 20 h, the solution is poured into water (700 mL) and extracted with EtOAc (300 mL). The organics are dried over Na2SO4, filtered, and concentrated. Purification by flash silica gel column chromatography (4:1 hexane / EtOAc to 1:1 hexane / EtOAc) affords the title compound as a red-brown solid.
Step 2. 1-(4-Chloro-1-pyrimidin-2-yl-1H-indol-3-yl)-2,2,2-trifluoroethanone
[0282]
[0283]A mixture of 1-(4-chloro-1H-indol-3-yl)-2,2,2-trifluoroethanone (10.1 g, 40.8 mmol), 2-chloropyrimidine (9.3 g, 81 mmol), cesium carbonate (26.6 g, 81.6 mmol), and 1,4-dioxane (80 mL) under N2 is warmed to 100° C. for 3 h. After cooling to rt, water (200 mL) is added. The precipita...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com