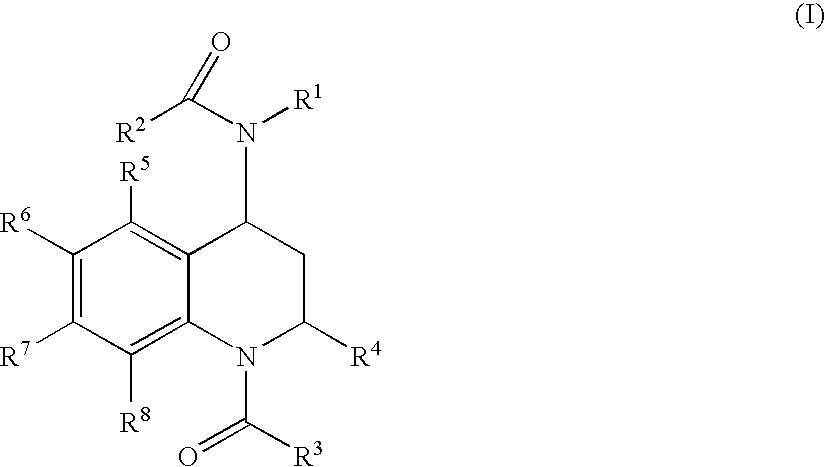
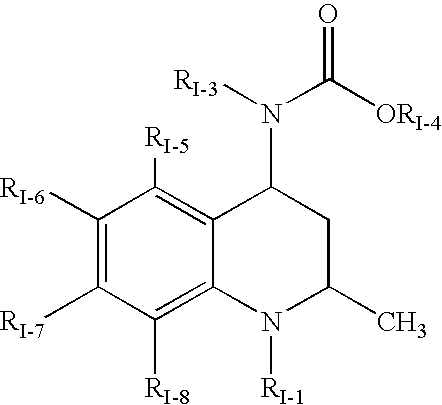
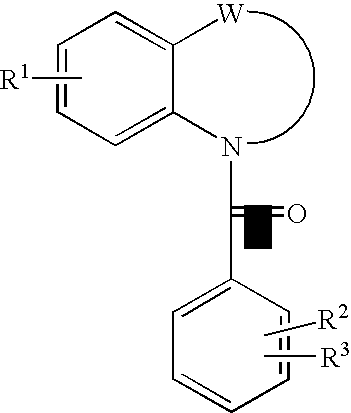
Quinoline Derivatives as CRTH2 Antagonists
a technology of derivatives and quinolines, applied in the field of quinoline derivatives, can solve the problems of limited use and low efficacy of these agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
synthesis examples
[0503] The following examples illustrate, without limiting it, the synthesis of particularly active compounds of formula (I) according to the invention.
[0504]1H-NMR spectra were recorded at 400 MHz. The residual solvent peak, usually chloroform (δH 7.27 ppm) or DMSO (δH 2.5 ppm) was used as internal shift reference. Analytical HPLC was run on a DIONEX Summit equipped with a diode array detector, using a Kromasil C-18 reversed-phase column and eluting with the following general system: acetonitrile with 0.1% v / v of acid formic in water with 0.1% v / v of acid formic (5:95 to 95:5 in a 13 minutes gradient). LC / MS studies were run on a Hewlett-Packard HPLC 1100 coupled to a Micromass mass spectrometer.
[0505] HPLC: the purity was obtained at 214 nm, and expressed as a percentage of areas (are of the considered peak vs total of the peak areas).
Preparation of Intermediates
Intermediate A
Cis-N-(2-Methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-N-phenyl-acetamide
[0506] Intermediate A correspo...
example 1
Cis-N-[2-Methyl-1-(thiophene-2-carbonyl)-1,2,3,4-tetrahydro-quinolin-4-yl]-N-phenyl-acetamide
[0545] R1: phenyl, R2: CH3, R3: thien-2-yl, R4: CH3, R5: H, R6: H, R7: H, R8: H.
[0546] To a solution of intermediate A (0.1 g), diisopropyl ethyl amine (0.05 g) in dioxane (1 ml), was added 2-thiophencarbonyl chloride (0.057 mg), under stirring, at room temperature for 3 hours. Then dichloromethane (2 ml) and water (2 ml) were added. The organic layer was separated and the solvent was removed under reduce pressure. The crude compound was crystallized in diethyl ether, filtered and dried to give a white solid (0.055 g, 39.6% yield).
[0547]1H NMR [CDCl3]: d 7.4-7.2 (m, 8H), 7.05 (m, 1H), 6.85 (t, 1H), 6.75 (t, 1H), 6.7 (d, 1H), 5.5 (m, 1H), 4.7 (m, 1H), 2.3 (m, 1H), 2.0 (s, 3H), 1.6 (s, 1H), 1.1 (d, 3H).
[0548] HPLC: rT: 10.15, 100% purity
[0549] MS positive ESI: m / z (m+H)+=391
example 2
Cis-N-(1-Benzoyl-2-methyl-1,2,3,4-tetrahydro-quinolin-4-yl)-N-phenyl-acetamide
[0550] R1: phenyl, R2: CH3, R3: phenyl, R4: CH3, R5: H, R6: H, R7: H, R8: H.
[0551] The titled compound was prepared according to protocol A, using intermediate A and appropriate reactants.
[0552]1H NMR [CDCl3]: d 7.4-7.15 (m, 12H), 6.9 (t, 1H), 6.5 (d, 1H), 5.6 (m, 1H), 4.8 (m, 1H), 2.3 (m, 1H), 2.0 (s, 3H), 1.6 (s, 1H), 1.1 (d, 3H).
[0553] HPLC: rT: 10.15, 100% purity
[0554] MS positive ESI: m / z (m+H)+=385
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com