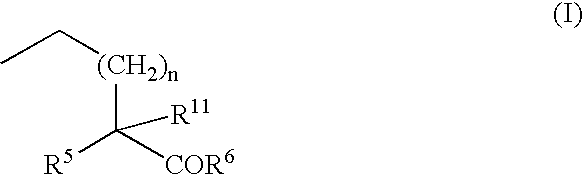Agent for treating Parkinson's disease comprising astrocyte function-improving agent as active ingredient
a technology of astrocyte function and active ingredient, which is applied in the field of parkinson's disease treatment, can solve the problems of insufficient therapeutic benefit and decline of drug effect of antiparkinsonian drugs at presen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Improvement Effect of the Compound of the Present Invention in Experimental Model of Parkinson's Disease Induced by Administration of MPTP
[0067] Male C57BL / 6 mice (body weight: 20 to 28 g) were divided into groups each having 6 to 12 animals. Without anesthetizing, MPTP (10 mg / kg; 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride) was intraperitoneally administered to the mice 4 times at intervals of 1 hour (Brain Res., 824: 224-231 (1999)). To the models thus prepared, Compound A of the present invention ((R)-2-propyloctanoic acid) was administered after 1, 6, 24 and 48 hours. Three days after the final administration, striata of the mice were collected. After weighing, the striata were immediately frozen and stored. Then, dopamine content and DOPAC (3,4-dihydroxypyenylacetate) content were measured by HPLC in a conventional manner and evaluated. Table 1 shows the results.
[0068] Dunnett's multiple comparison test (both sides) was performed on the basis of the data of the...
formulation example 1
Preparation of Capsules
[0072] (R)-2-propyloctanoic acid (1 g) was encapsulated into gelatin capsules to obtain 10 capsules each containing 100 mg of the active ingredient.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Content | aaaaa | aaaaa |
| Stiffness | aaaaa | aaaaa |
Abstract
- R5, R6, R11 and n are defined in the specification.
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com