Modified il-2 molecules and uses thereof
An IL-2, molecular technology, applied in the field of modified IL-2 molecules, which can solve problems such as side effects, chemotherapeutic drug resistance, and cancer cells cannot be completely removed from the patient's body.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
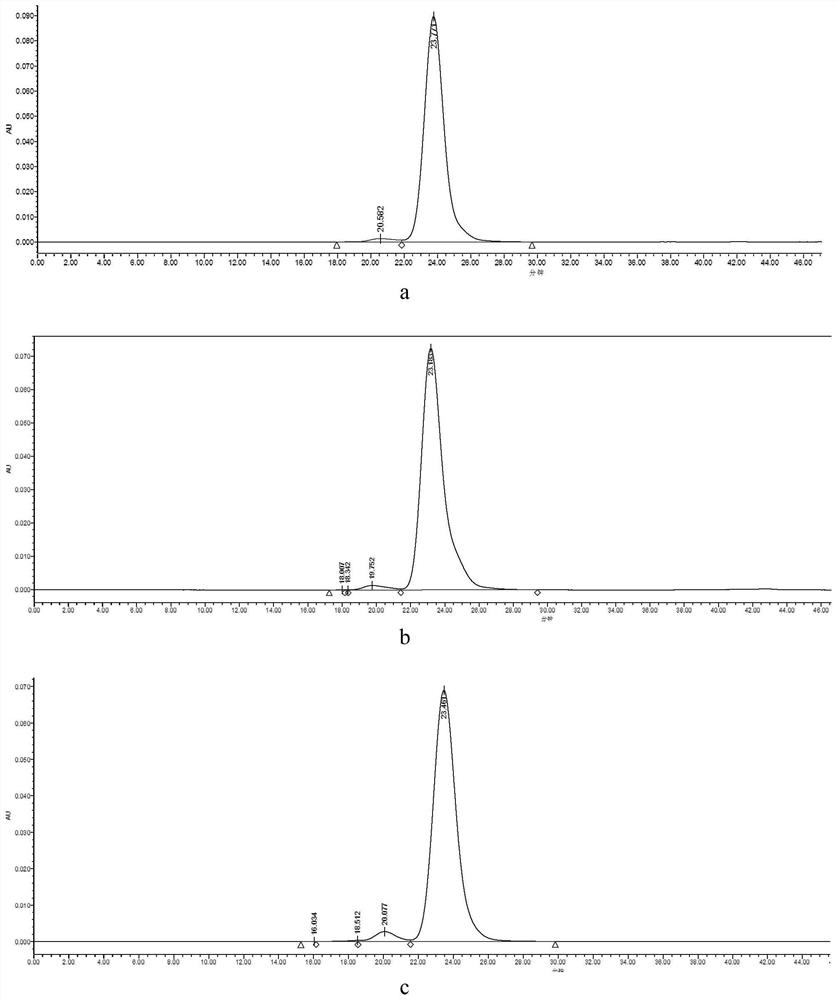
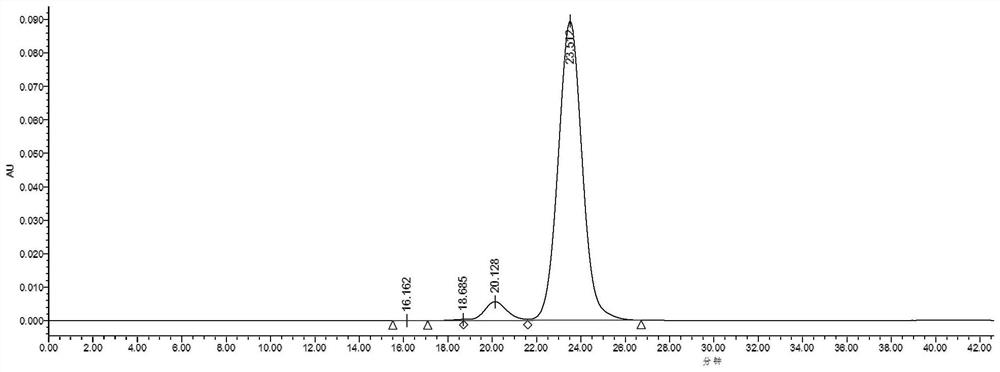
Image
Examples
Embodiment 1
[0095] Example 1 Preparation of IL-2 / 15 chimera from mammalian cells
[0096] 1.1 Synthesis and construction of expression plasmids
[0097] Entrust Suzhou Jinweizhi Biotechnology Co., Ltd. to synthesize IL-2 / 15 chimera 1 (SEQ ID NO.14), sushi-hIgG1Fc (SEQ ID NO.19), sushi-hIgG4Fc (SEQ ID NO.21), sushi- The target gene fragments of the 4 proteins of mIgG2aa.1Fc (SEQID NO.22) were cloned between the EcoRI and HindIII sites of the pZD vector to obtain 4 expression plasmids, numbered respectively PM619, PM432, PM657, and PM599. On the basis of PM619, a point mutation was carried out according to the method described in "Molecular Cloning", and the 115th position (corresponding to the 121st position of the natural IL-2 molecular amino acid sequence) phenylalanine (F) was mutated into tryptophan (W), Further, the plasmid of IL-2 / 15 chimera 2 (SEQ ID NO.23) was obtained, and the plasmid number was PM824. Then the synthesized or constructed plasmids were transformed into DH10B, seq...
Embodiment 2
[0121] Embodiment 2 prepares IL-2 / 15 chimera from escherichia coli
[0122] 2.1 Synthesis and construction of expression plasmids
[0123] Entrusted Suzhou Jinweizhi Biotechnology Co., Ltd. to synthesize the IL-2 / 15 chimera 1 gene fragment (SEQ ID NO.26), and clone it into the pET41a vector between the NdeI and XhoI sites to obtain the expression plasmid, numbered 1187.
[0124] 2.2 Expression and purification of IL-2 / 15 chimera in Escherichia coli
[0125] According to the operation method mentioned in "Molecular Cloning", the plasmid was extracted, and the expression strain BL21(DE3) was transformed. The expression of IL-2 / 15 chimera 1 was carried out according to the routine method of protein prokaryotic expression. Afterwards, through techniques such as classical denaturation techniques and chromatography (see for example: Yunier Rodríguez- et al, Preparative Biochemistry and Biotechnology, 47:9, 889-900) were purified to obtain a relatively pure chimeric protein.
Embodiment 3I
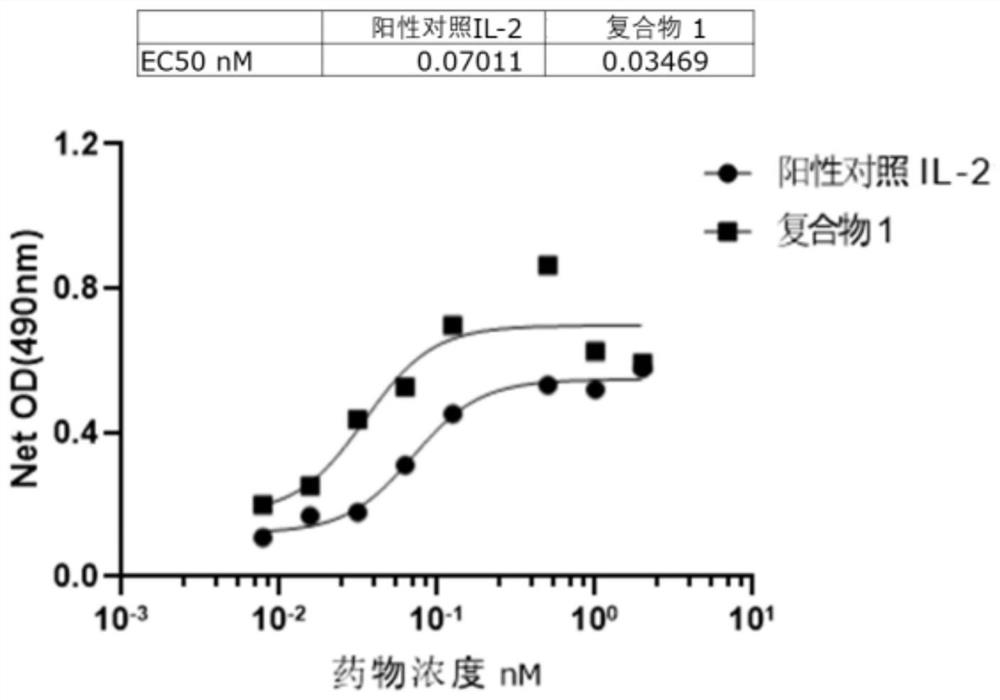
[0126] Example 3 Determination of the Binding Ability of IL-2 / 15 Chimera to IL2Rα and IL2 / 15Rβ
[0127] Biolayer interferometry (biolayer interferometry, BLI) was used to measure the affinity between the target protein and the receptor. For the method, see (Estep, P et al., High throughput solution Based measurement of antibody-antigen affinity and epitope binning.MAbs, 2013.5(2): p.270-8) to proceed. The receptor proteins IL-15Rα-his, IL2Rα-his and IL2 / 15Rβ-Fc / Fc used in the experiment are all produced by our company, and no IL2Rα-binding IL-2 derivatives and no IL2Rα-binding IL-2 complexes are based on the patent publication The record preparation of CN111018961A. The IL-15(N72D) / sushi-hIgG1Fc complex was prepared according to the literature (K.-p.Han et al. / Cytokine 56(2011)804-810). The buffer formulation was 10mM HEPES, 150mM NaCl, 3mM EDTA, 0.1% BSA and 0.05% tween20; the receptor proteins were all immobilized on the corresponding sensors in advance, and then according...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap