Improvement in locomotor activity and increase in longevity of late infantile neuronal ceriod lipofuscinosis subjects by gemfibrozil
A technology of gemfibrozil and neurons, applied in the field of treatment of neurodegenerative diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Materials and methods:
[0050] Reagents and antibodies: Reagents for the TUNEL assay on frozen brain sections were purchased from EMDMillipore (Billerica, MA) and experiments were performed according to the manufacturer's instructions. Blocking buffer and secondary antibodies (IRDye 700 or IRDye 800 labeled) for western blotting (IB) were purchased from Licor (Lincoln, Nebraska). Secondary antibodies (FITC or Cy5-labeled) for immunohistochemistry (IHC) were purchased from Jackson ImmunoResearch (West Grove, PA). The sources of the primary antibodies used in this study and their applications and dilutions are listed in Table 1.
[0051] Animals: Animal maintenance and experimentation were in accordance with the requirements of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee (IACUC) of Rush University Medical Center, Chicago, IL. Animals exhibiting mild seizures and tremors were fed and watered by animal...
Embodiment 2
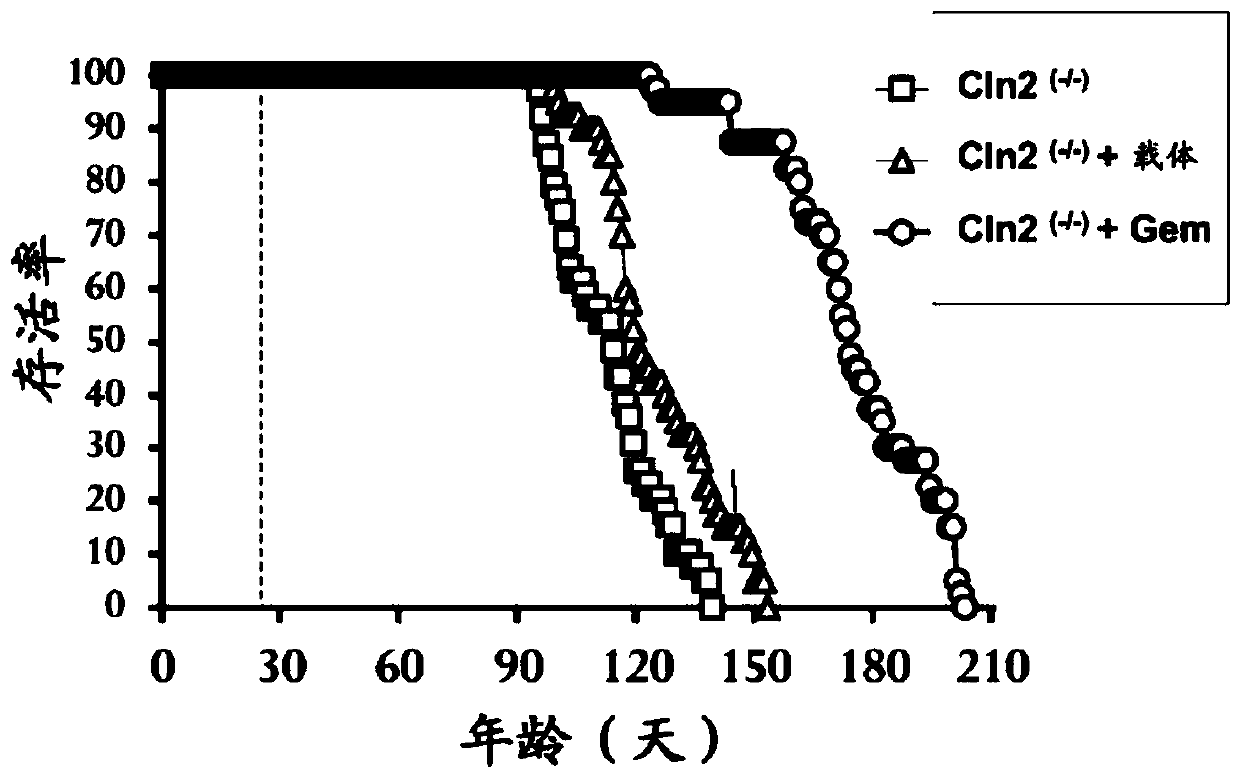
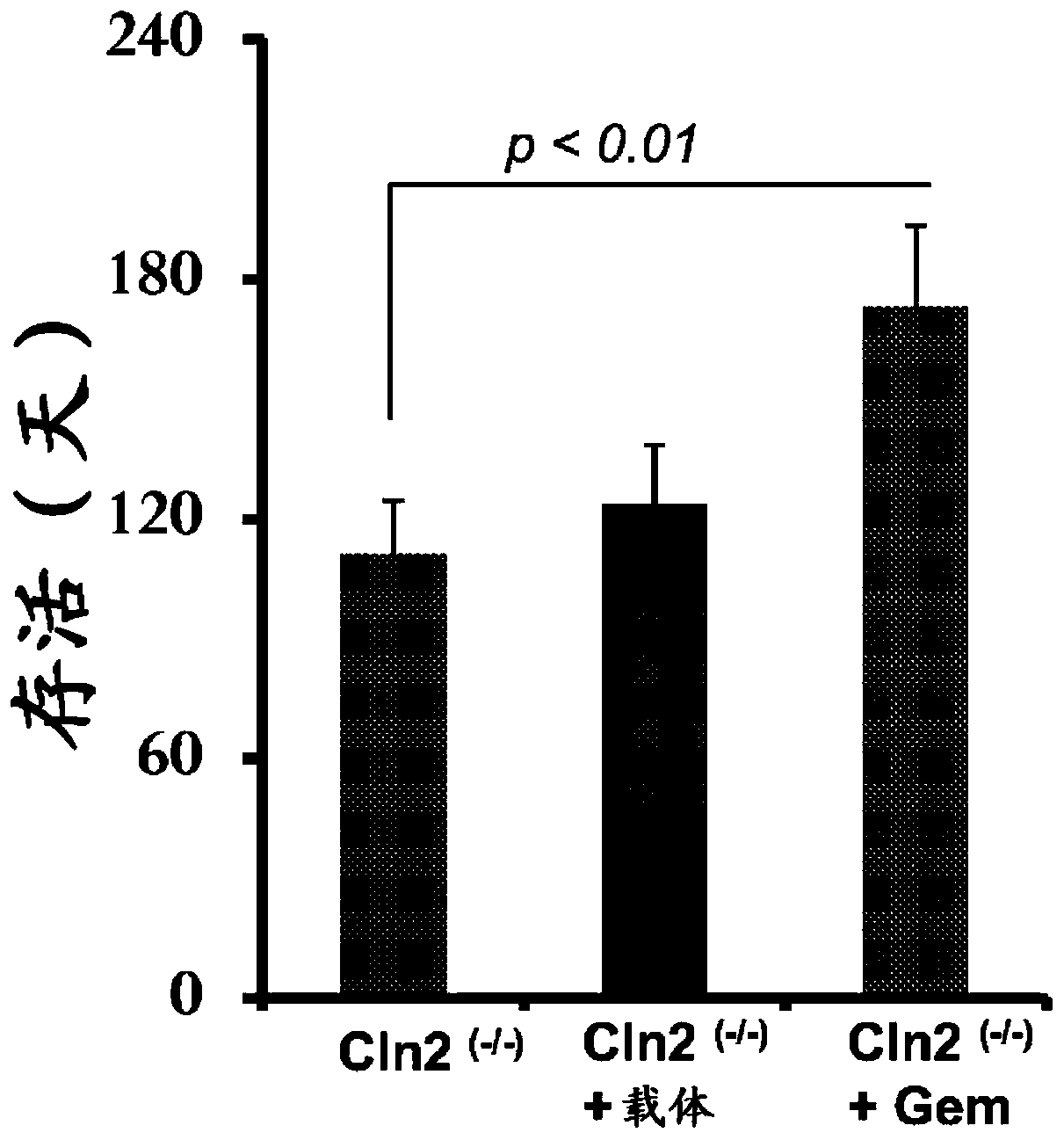
[0058] Example 2: Gem treatment prolongs Cln2 (- / -) Mouse lifespan: Cln2 (- / -) Mice are an important animal model for testing new therapeutic approaches against LINCL (Cabrera-Salazar et al., 2007; Sleat et al., 2008; Chang et al., 2008; Sleat et al., 2004). Typically, LINCL progresses rapidly, ending in death between 8 and 10 years of age (Sohar et al., 1999; Sleat et al., 1997). Similarly, Cln2 (- / -) Mice also died within 140 days (Sleat et al., 2004). Therefore, we first examined whether oral gem treatment could increase Cln2 (- / -) mouse lifespan. Earlier we have shown that gem enters the CNS after oral administration (Dasgupta et al., 2007). From 4 weeks of age, mice were treated daily with gem (7.5 mg / kg body weight / day) by gavage. Since the gem is dissolved in 0.1% methylcellulose, a set of Cln2 (- / -) Mice also received 0.1% methylcellulose as a vehicle. Untreated Cln2 (- / -) Male and female mice died from 95 days, and within 137 days, all Cln2 (- / -) Mice died (...
Embodiment 3
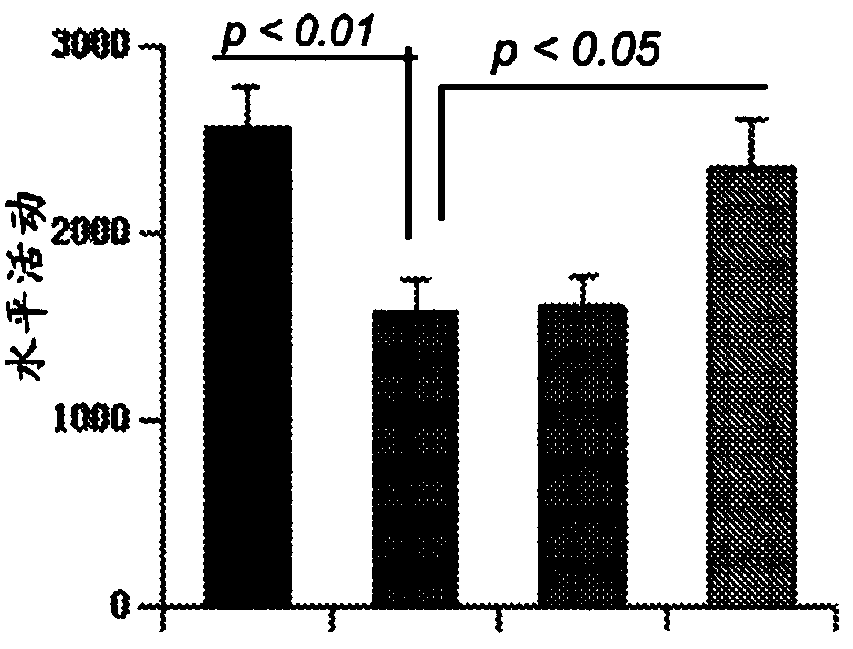
[0059] Example 3: gem treatment improves Cln2 (- / -) Locomotor behavior in mice: In addition to increased lifespan, another therapeutic goal for neuroprotection in LINCL patients is to reduce dysfunction. So to check if the gem not only increases lifespan but also improves Cln2 (- / -) For the locomotor behavior of mice, we monitored locomotor activity. Locomotor activity was monitored 8 weeks after gem treatment. Compared with WT mice, Cln2 (- / -) The horizontal activity of mice ( Figure 2A ),sport time( Figure 2B ), exercise times ( Figure 2C ), the total distance traveled ( Figure 2D ) and stereotype counts ( Figure 2E ) was significantly reduced. On the other hand, Cln2 (- / -) The resting time of mice was longer than that of WT mice ( Figure 2F ). However, oral administration of the gem significantly improved Cln2 (- / -) The locomotor activity of mice ( Figure 2A -F).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com