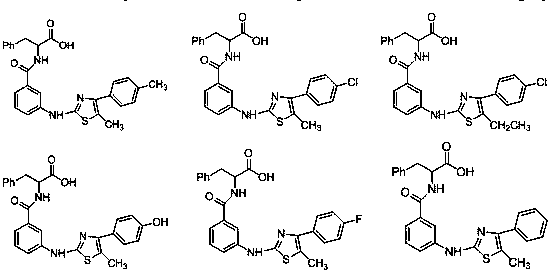
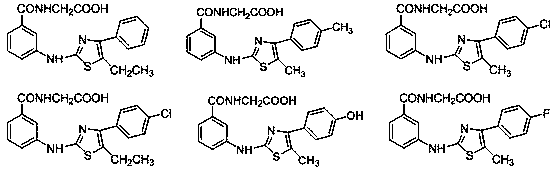
Thiazole aminobenzamide acetic acid derivative and application thereof
An alkyl and benzyl technology, applied in the field of medicinal chemistry, can solve problems such as ineffective drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1 (3-((5-methyl-4-phenylthiazol-2-yl) amino) benzoyl) glycine
[0057] Step a:
[0058]
[0059] Add 11.4341 g (0.12 mol) of ammonium thiocyanate and 20 mL of acetone into a 100 mL oblique-necked reaction flask equipped with mechanical stirring and a condenser, and stir evenly through mechanical stirring. 16.8034 g (0.13 mol) of benzoyl chloride was added dropwise (dropped in 10 min), and the solution changed from clear to white turbid. Heat to reflux, add 14.1147 g (0.10 mol) m-aminobenzoic acid in 4 batches, monitor the reaction process by TLC (ethyl acetate:petroleum ether = 4:1), and complete the reaction in 8 h. Cool, filter, and dry the obtained solid to obtain 28.0041 g of light yellow powder 3-(3-benzoylthioureido)benzoic acid, m.p. 184 ~ 186 ℃.
[0060] Add 0.9913 g (0.12 mol) of 3-(3-benzoylthioureido)benzoic acid and 33 mL of 10% NaOH into a 100 mL oblique-necked reaction flask with a condenser, the measured pH=13, magnetic stirring, and heati...
Embodiment 2
[0070] Example 2 (3-((5-methyl-4-(p-tolyl) thiazol-2-yl) amino) benzoyl) glycine
[0071]
[0072] The operation was the same as above to obtain 0.0934 g of brown powder, yield 61.28%, m.p.225-228°C. 1 H NMR (DMSO-D 6 , 400MHz), δ : 2.36 (s, 3H, CH 3 ), 2.41 (s, 3H, CH 3 ), 3.93(d, J = 8.0 Hz, 2H, NHCH 2 ), 7.26-8.08 (m, 8H, 2×C 6 h 4 ), 8.82(t, J = 8.0 Hz, 1H, CONH), 10.49 (s, 1H, COOH).
Embodiment 3
[0073] Example 3 3-((4-(4-chlorophenyl)-5-methylthiazol-2-yl)amino)benzoyl)glycine
[0074]
[0075] The operation was the same as above to obtain 0.09492 g of yellow powder, yield 59.18%, m.p.238-240°C. 1 H NMR (DMSO-D 6 , 400MHz), δ : 2.43 (s, 3H, CH 3 ), 3.91(d, J = 8.0 Hz, 2H, NHCH 2 ), 7.26-8.08 (m, 8H, 2×C 6 h 4 ), 8.75 (t, J = 8.0 Hz, 1H, CONH), 10.25 (s, 1H, COOH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com