Crown Ether Extraction and Separation of Lithium Isotopes
A lithium isotope and extraction technology, applied in the chemical industry, can solve the problems of difficult multi-stage countercurrent and continuous production, no relatively mature production process, and the multi-stage cascade extraction process has not yet been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
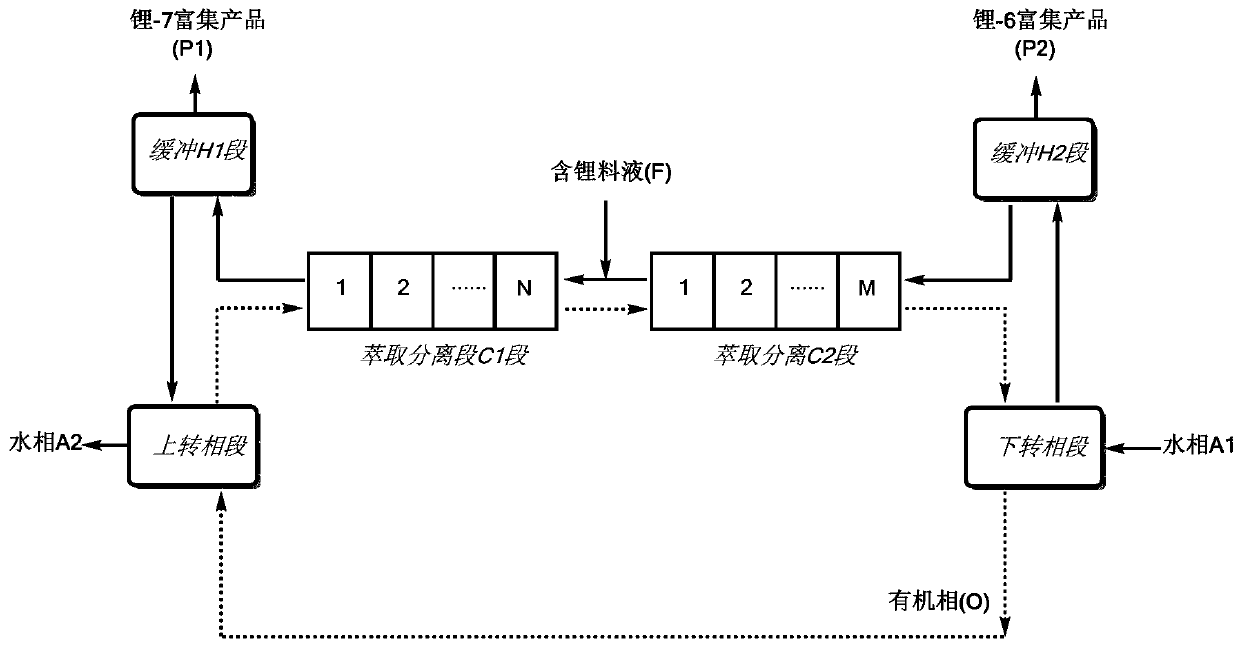
[0080] Connect the pipelines according to the process diagram, and a total of 20 centrifugal extractors are used in the extraction and separation C1 section and the extraction and separation C2 section. The buffer H1 section contains a liquid storage tank with a volume of 0.025m 3 ;The buffer H2 section contains a liquid storage tank with a volume of 0.002m 3 .
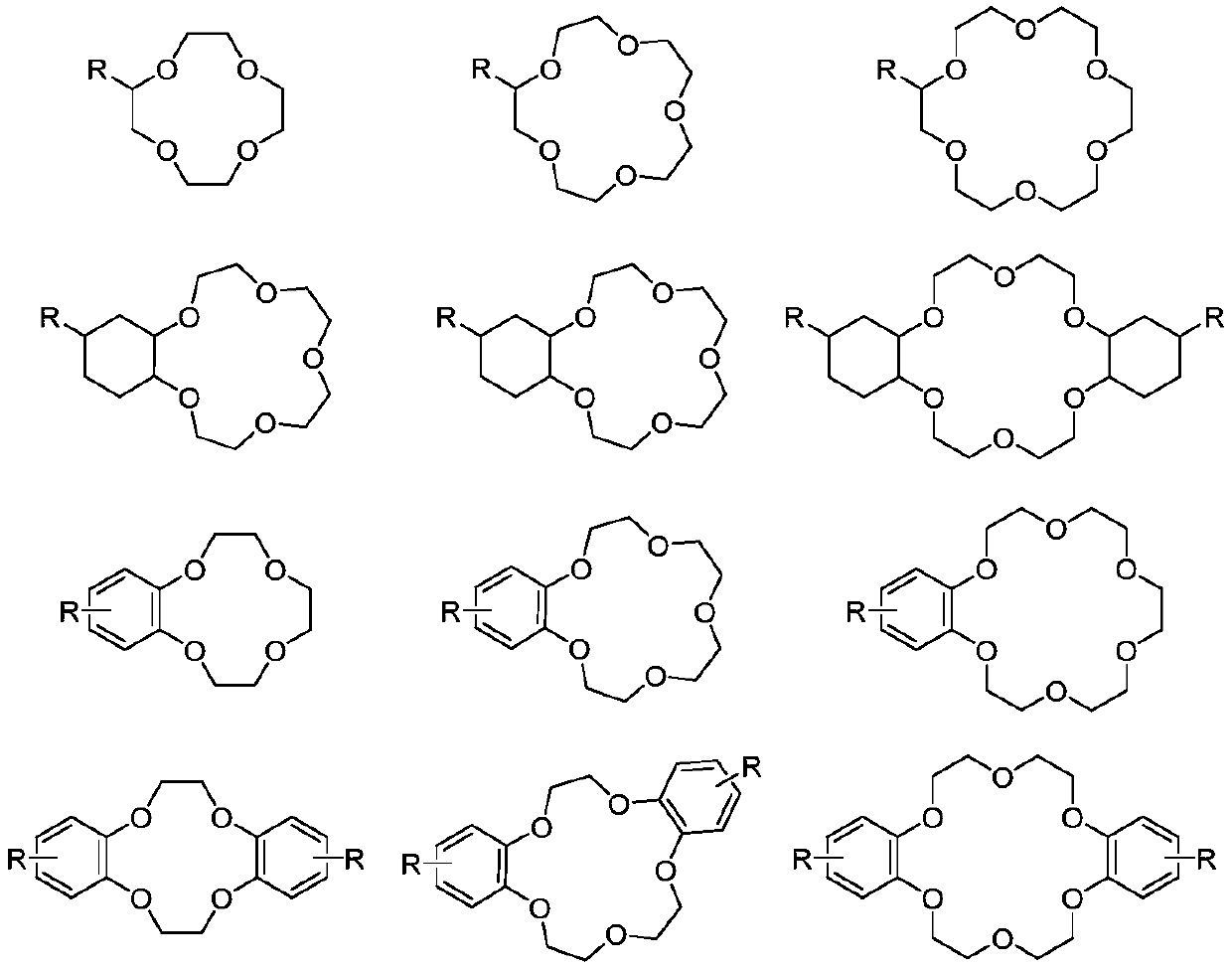
[0081] Organic phase: the extractant is dicyclohexyl 18-crown-6, the diluent is dichlorobenzene, and the separation coefficient of the single-stage extraction of the organic phase is 1.031.
[0082] Lithium-containing feed solution (F): LiI aqueous solution, 2.5mol / L, wherein the abundance of lithium-6 is 7.48%, and the abundance of lithium-7 is 92.52%.
[0083] Through the feeding pump, the organic phase, aqueous phase and feed liquid are continuously added, and the entire extraction process is run. After 65 hours, the system reaches equilibrium, and the enriched product is continuously separated and obtained:
[...
Embodiment 2
[0086] The technical process and the feed liquid all adopt the parameters in Example 1, only changing the volume of the storage tank in the buffer H1 section to 0.050m 3 . Enrichment products that are different from the abundance values in Example 1 can be continuously obtained:
[0087] The lithium-7 abundance value of the lithium-7 enriched product (P1) is 93.27%, and the extraction and separation C1 stage efficiency is 95%; the lithium-6 abundance value of the lithium-6 enriched product (P2) is 10.20% , The extraction and separation C1 stage efficiency is 95%.
Embodiment 3
[0089] Connect the pipelines according to the process diagram, use 25 centrifugal extractors for the extraction and separation C1 section, and 55 centrifugal extractors for the extraction and separation C2 section. The buffer H1 section and the buffer H2 section contain liquid storage tanks and temperature controllers.
[0090] Organic phase: the extractant is 4-tert-butylbenzo-15-crown-5, the diluent is kerosene, and the separation coefficient of the single-stage extraction of the organic phase is 1.032.
[0091] Lithium-containing feed solution (F): LiClO 4 Aqueous solution, 3.0mol / L, in which the abundance of lithium-6 is 7.49%, and the abundance of lithium-7 is 92.51%.
[0092] Through the feeding pump, the organic phase, aqueous phase and feed liquid are continuously added to run the entire extraction process. After the system reaches equilibrium, continuous separation and enrichment products are obtained:
[0093]The lithium-7 abundance value of the lithium-7 enriched ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com