Directional synthesis method for 21(S) argatroban
A technology of argatroban and directional synthesis, which is applied in the field of medicine and can solve problems such as low efficiency, high cost, and no industrial practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1) Add 200ml chloroform and 25g (0.061mole) (2R, 4R)-1-[N G -Nitro-L-arginyl]-4-methyl-2-piperidinecarboxylic acid ethyl ester hydrochloride, cooled to 5°C, then added 18.5g (0.18mole) triethylamine, and added dropwise 14.7g ( 0.061mole) (3S)-1,2,3,4-tetrahydro-3-methyl-8-quinolinesulfonyl chloride, stirred at room temperature for 3 hours, TLC tracking reaction, to (2R,4R)-1-[ N G -Nitro-L-arginyl]-4-methyl-2-piperidinecarboxylic acid ethyl ester hydrochloride disappeared, the reaction was completed, washed twice with 50ml of water, dried over anhydrous magnesium sulfate, evaporated to remove solvent, column chromatography Separation gave (2R,4R)-1-[N G -Nitro-N 2 -[(3S)-1,2,3,4-tetrahydro-3-methyl-8-quinolinesulfonyl]-L-arginyl]-4-methyl-2-piperidinecarboxylate VI 32.5g, the yield is 92.1%;
[0039] 2) Add 100ml of ethanol and 30g (0.052mole) of intermediate VI into a 500ml four-necked reaction flask, add 100ml of 1N NaOH under stirring, stir at room temperature f...
Embodiment 2
[0043] Add 1g of 21(S) Argatroban into 150ml of distilled water, stir, and heat up to 95°C within 0.5 to 1.0 hours. At this time, all the solids are dissolved, and then cool down. 80°C, then drop to 50°C within 1 hour, then drop to 0°C within 2 hours. Placed at 0° C. for 8 hours until crystallization, then filtered, washed with water, and vacuum-dried at a temperature of 50 to 60° C. to obtain 0.65 g of 21(S) argatroban monohydrate, with a water content of 3.4% ( Karl Fischer method).
Embodiment 3
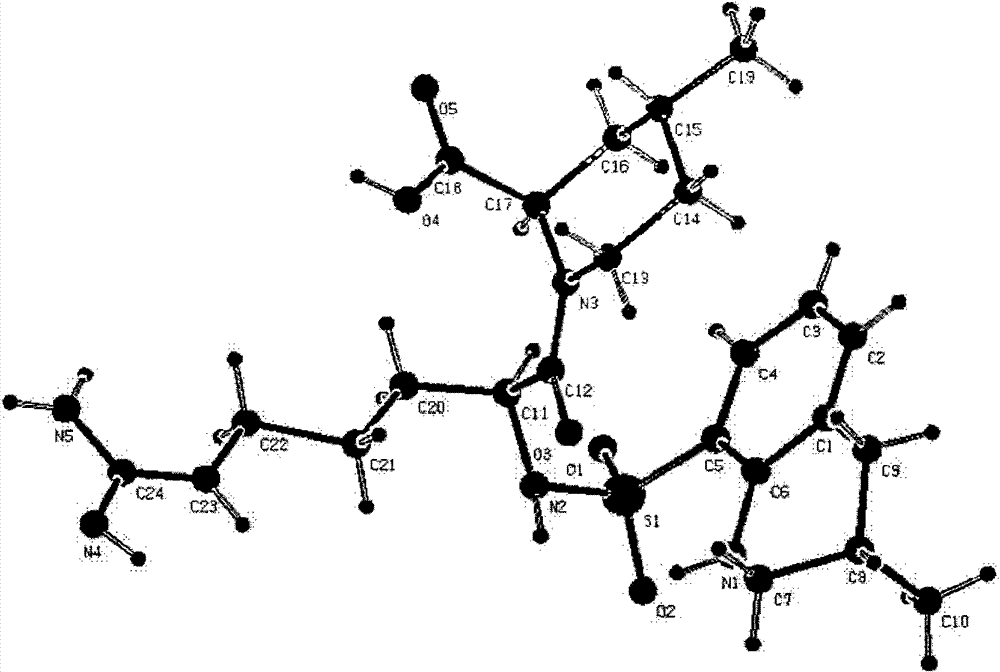
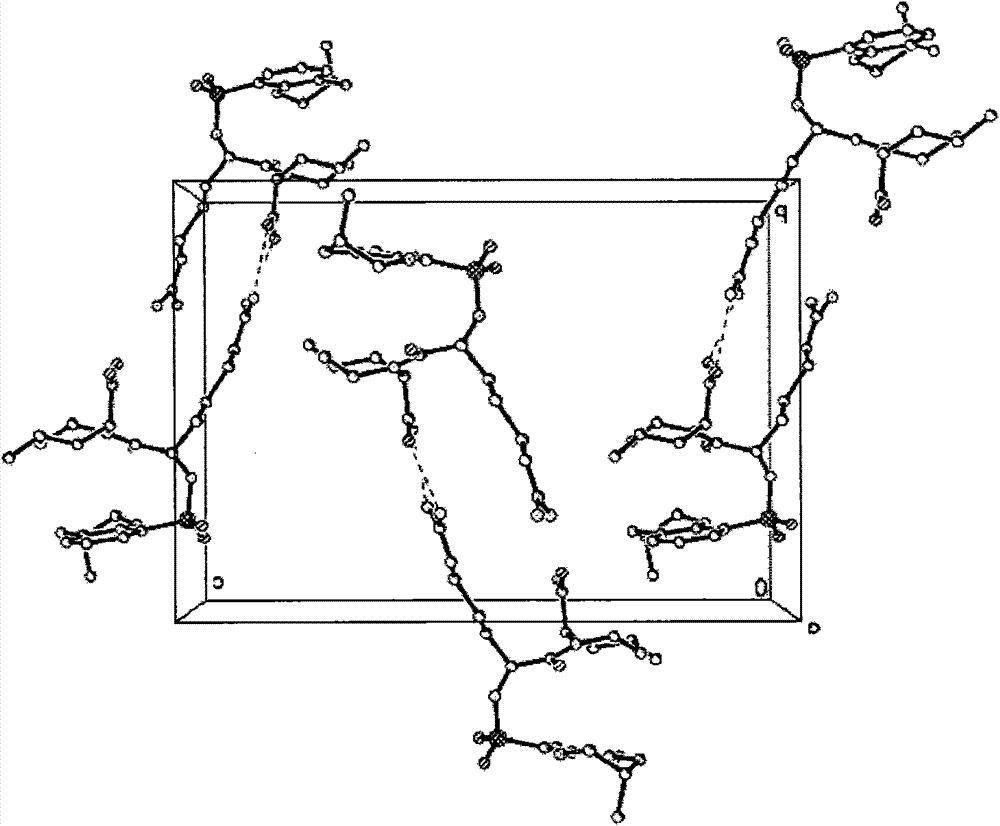
[0045] 1g of anhydrous 21(S) argatroban VIII was recrystallized with distilled water to obtain crystal form I, and X-ray diffraction of single crystal and polycrystalline powder confirmed that its absolute configuration was S body at position 21 (see figure 2 ), crystal form I is an orthorhombic crystal system, P2(1)2(1)2(1) space group (No. 19 space group). Cell parameters: α=β=γ=90°C (see image 3 ). Form I polycrystalline powder X-ray diffraction data are consistent with single crystal X-ray diffraction simulation data (see Figure 4 ,Table 1).
[0046] Table 1 Comparison of X-Diffraction Data of Single Crystal and Polycrystalline Powder of 21(S) Argatroban Form I
[0047]
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com