Ruthenium mixed-polypyridyl complex, preparation thereof and use as antitumor drug
A technology of ruthenium polypyridine and complexes, applied in the field of medicinal chemistry, can solve problems such as enhancing the water solubility of complexes, and achieve the effects of strong inhibitory activity and strong water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 [Ru(bpy) 2 (3′-DOA)]Cl 2 (I) Preparation
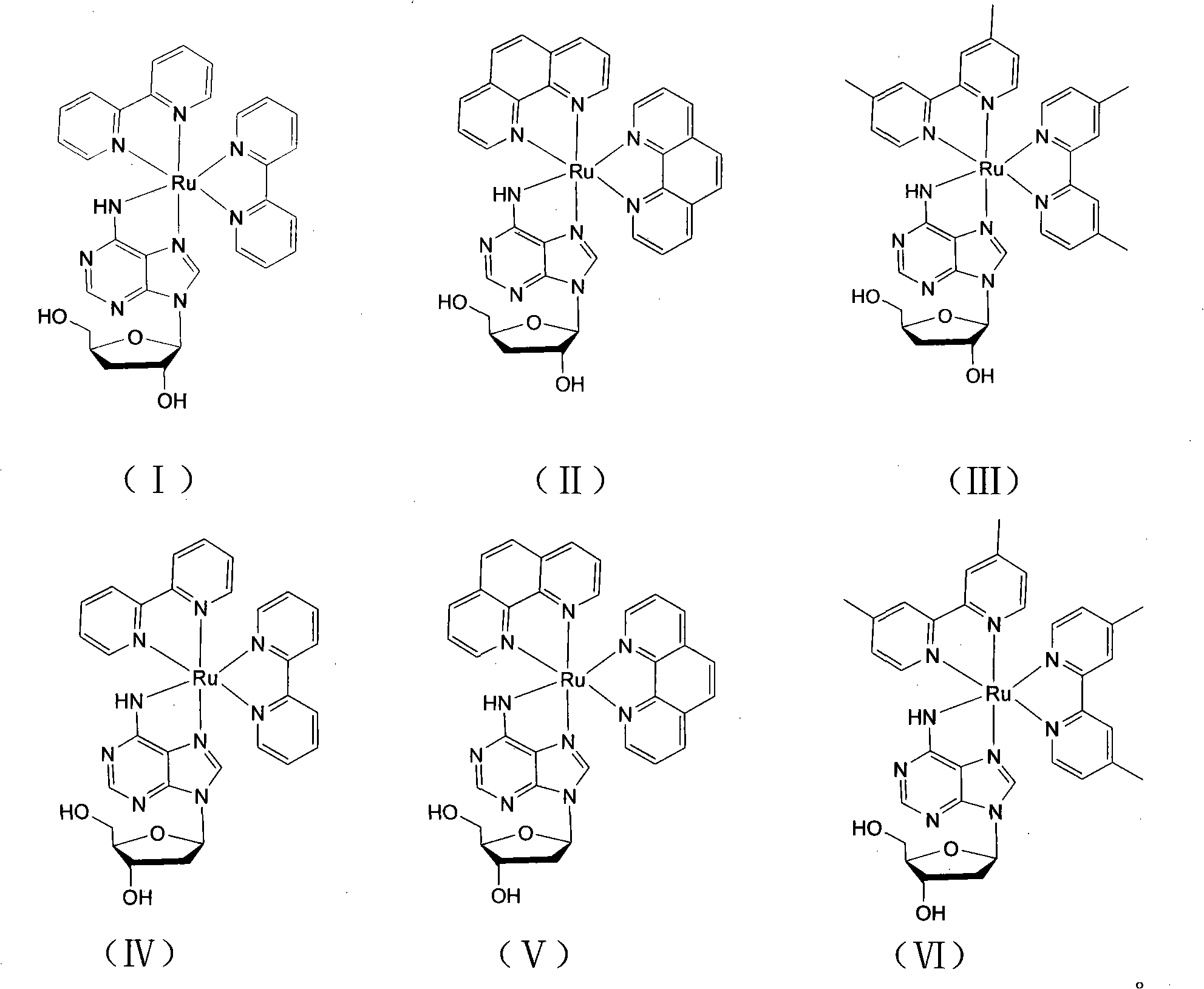
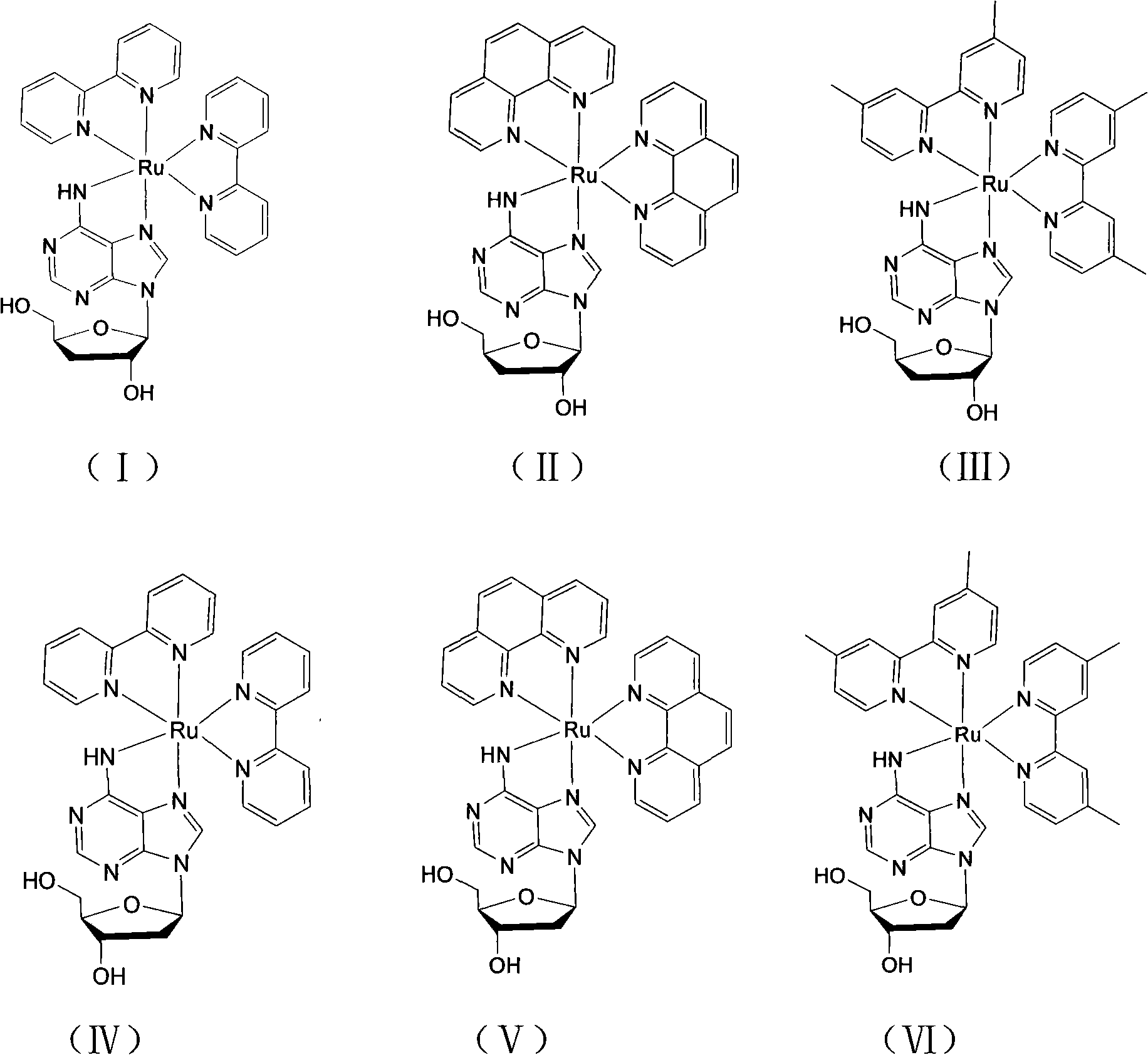
[0034] [Ru(bpy) of the present embodiment 2 (3′-DOA)]Cl 2 With 3'-deoxyadenosine as the main ligand and bipyridine (bpy) as the auxiliary ligand, its structural formula is shown in formula (I), and its preparation method is as follows:
[0035]
[0036] 1.1 cis-[Ru(bpy) 2 Cl 2 ]·2H 2 Synthesis of O
[0037] Ruthenium trichloride (RuCl 3 ·nH 2 O) (1.56g, 6mmol), bipyridine (1.87g, 12mmol) and lithium chloride LiCl (1.68g, 28mmol), dissolved in 15ml of DMF, heated to reflux for 8 hours under the protection of argon, cooled to room temperature, added 50ml Acetone, shake well, freeze overnight in the refrigerator, filter to obtain purple-black microcrystals, wash with ice water and acetone until nearly colorless, and dry to obtain [Ru(bpy) 2 Cl 2 ]·2H 2 O, yield 71% (calculated as bipyridyl).
[0038] 1.2 [Ru(bpy) 2 (3′-DOA)]Cl 2 preparation of
[0039] cis-[Ru(bpy) 2 Cl 2 ]·2H 2 O (312.0mg, 0.6mm...
Embodiment 2
[0041] Embodiment 2 [Ru(phen) 2 (3′-DOA)]Cl2 Preparation of (II)
[0042] [Ru(phen) of the present embodiment 2 (3′-DOA)]Cl 2 With 3'-deoxyadenosine as the main ligand and phenanthroline as the auxiliary ligand, its structural formula is shown in formula (II), and its preparation method is as follows:
[0043]
[0044] 2.1 cis-[Ru(phen) 2 Cl 2 ]·2H 2 Synthesis of O
[0045] Ruthenium trichloride (RuCl 3 ·nH 2 (2) (1.56g, 6mmol), phenanthroline (2.16g, 12mmol), lithium chloride LiCl (1.68g, 28mmol), be dissolved in 10mlDMF, after refluxing 8 hours under protection of argon, treat that reactant is cooled to At room temperature, add 50ml of acetone to the reactant, refrigerate overnight, filter to obtain purple-black crystals, wash with ice water and acetone until nearly colorless, and dry in vacuo to obtain [Ru(phen) 2 Cl 2 ]·2H 2 O, yield 72% (yield calculated with phenanthroline).
[0046] 2.2 [Ru(phen) 2 (3′-DOA)]Cl 2 preparation of
[0047] will cis-[Ru(phe...
Embodiment 3
[0049] Embodiment 3 [Ru(dmbpy) 2 (3′-DOA)]Cl 2 Preparation of (III)
[0050]
[0051] [Ru(dmbpy) of the present embodiment 2 (3′-DOA)]Cl 2 With 3'-deoxyadenosine as the main ligand and dimethylbipyridine (dmbpy) as the auxiliary ligand, its structural formula is shown in formula (III), and its preparation method is as follows:
[0052] 3.1 cis-[Ru(dmbpy) 2 Cl 2 ]·2H 2 Synthesis of O
[0053] Ruthenium trichloride (RuCl 3 ·nH 2 O) (1.56g, 6mmol), 4,4'-dimethyl-2,2'-bipyridine (2.21g, 12mmol) and lithium chloride LiCl (1.68g, 28mmol), dissolved in 15ml DMF, argon After heating and refluxing under protection for 8 hours, cool to room temperature, add 50ml of acetone, shake well, put in the refrigerator to freeze overnight, filter to obtain purple-black microcrystals, wash with ice water and acetone until nearly colorless, and dry to obtain [Ru(dmbpy ) 2 Cl 2 ]·2H 2 O, yield 70.0% (calculated in dimethylbipyridine).
[0054] 3.2 [Ru(dmbpy) 2 (3′-DOA)]Cl 2 prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com