Fusion protein for seralbumin and interferon
A technology of serum albumin and human serum albumin, applied in the field of fusion proteins, can solve the problems of short plasma half-life, increased patient suffering and treatment costs, as long as several months or even years, and achieve the effect of prolonging the plasma half-life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
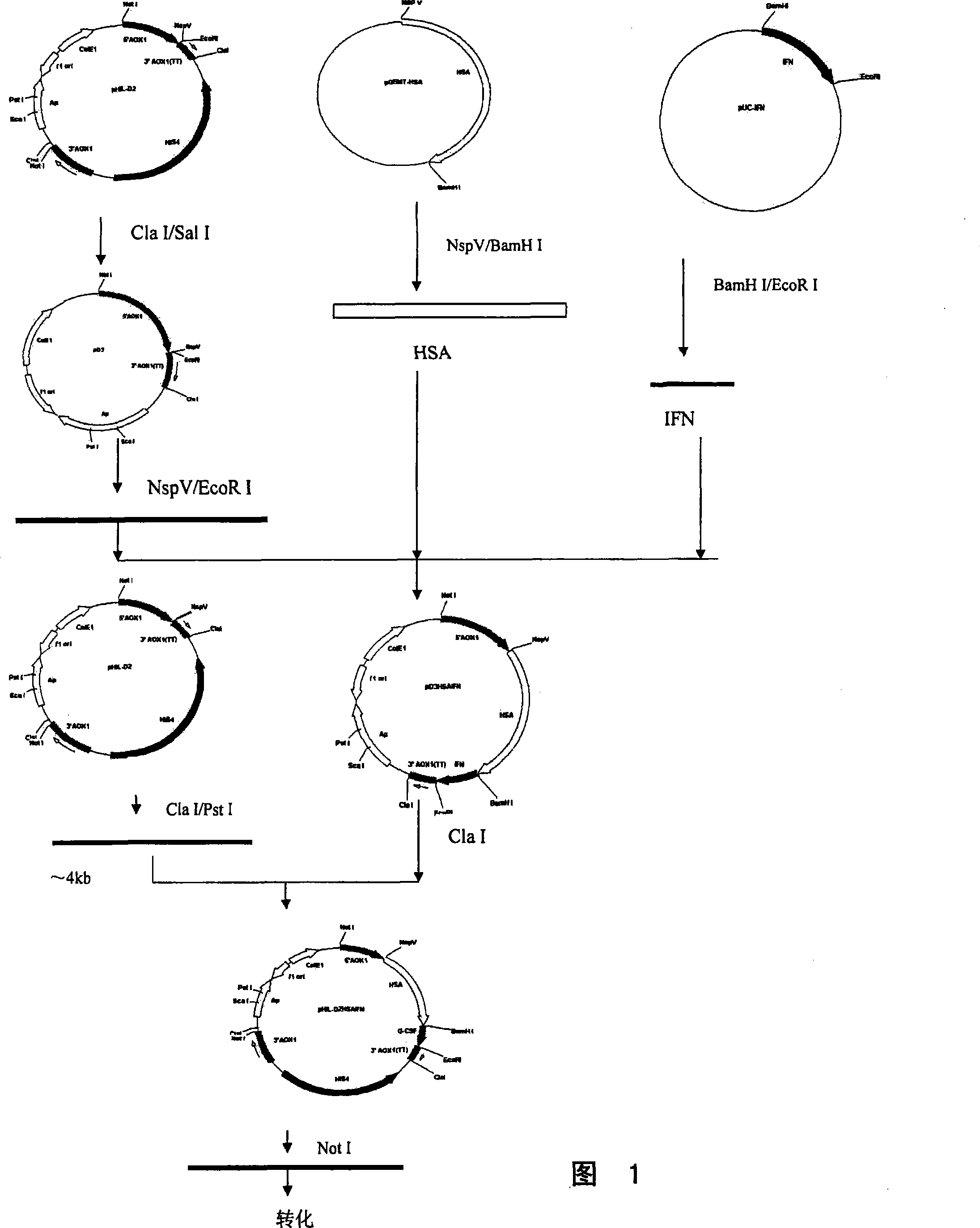
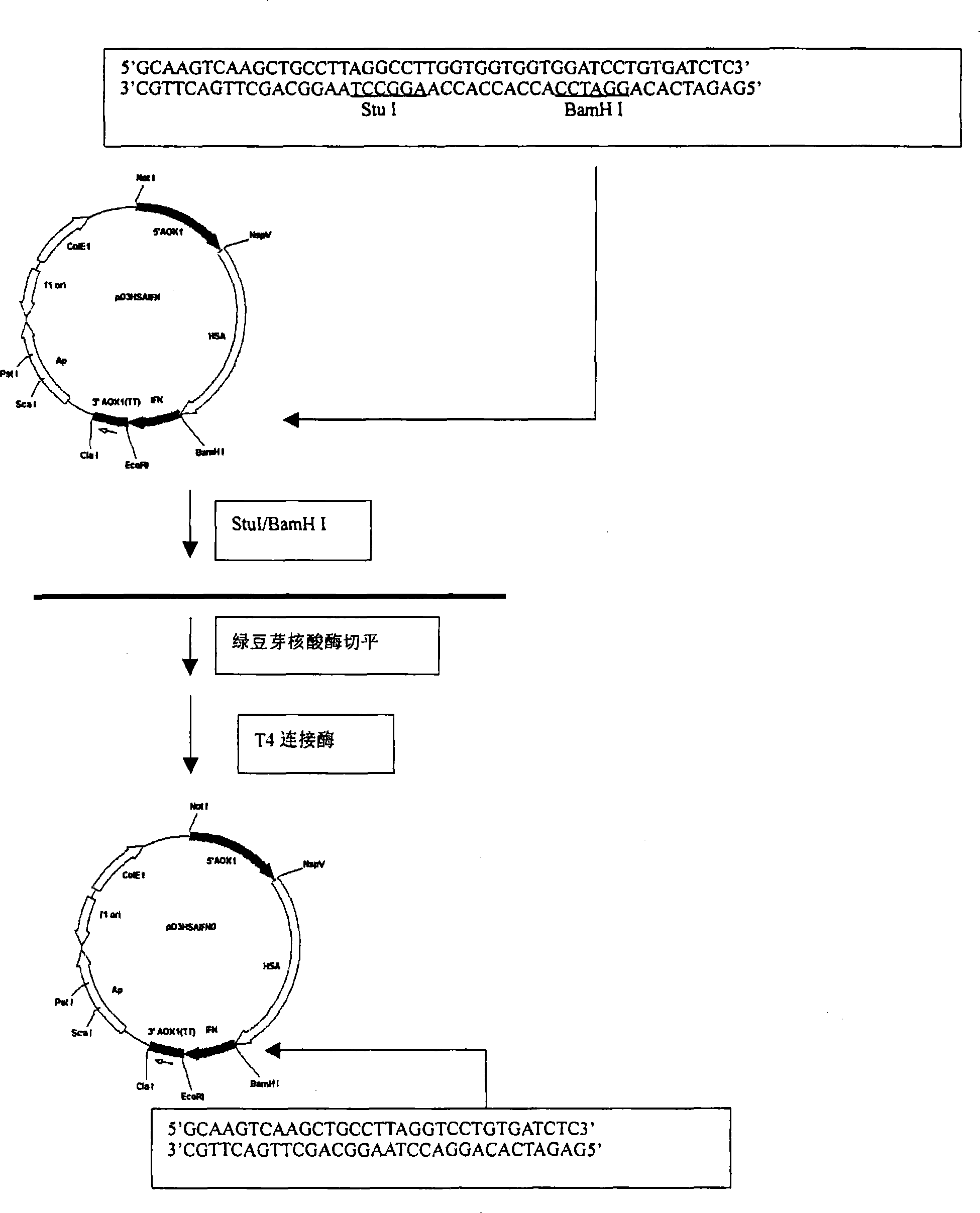
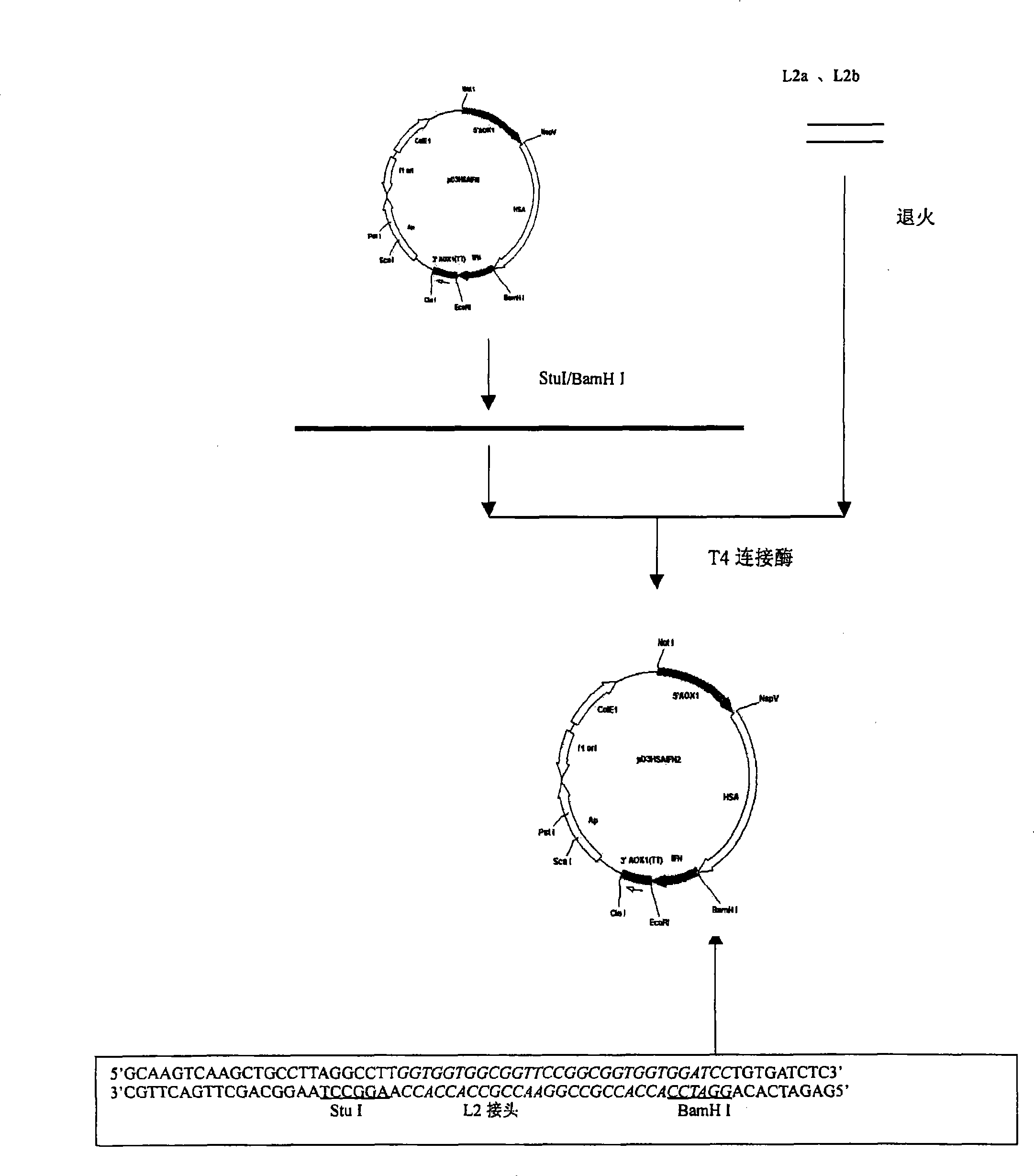
[0045] Example 1: Cloning of HSA cDNA
[0046] The HSA cDNA with the signal peptide and propeptide coding sequences was obtained from the human liver fetal cDNA library (purchased from Clontech Laboratories Inc.USA) by PCR method, and the primers HSA1 and HSA2 used were synthesized with an oligonucleotide synthesizer, wherein the primers In addition to the 3' end sequence of the HSA gene, HSA2 also added a sequence encoding a GlyGlyGlyGlySer linker peptide after the 3' end.
[0047] HSA1:5'GC TTCGAA ACCATGAAGTGGGTAACCTTTATTTCCCT3'
[0048] HSA2: 5'TA GGATCC ACCACCACCAAGGCCTAAGGCAGCTTGACTTGC3'
[0049] The PCR conditions were: 1 μl of human hepatic fetal cDNA library, 3 μl of 20 μmol / L HSA1 and HSA2 primers, 10 μl of 2 mmol / L dNTP, 10 μl of 10X reaction buffer, 5 U of TaqplusI DNA polymerase in 100 μl reaction system. Using a 9600 PCR instrument from Perk-Elmer, the PCR conditions were denaturation at 94°C for 1 minute, annealing at 52°C for 1 minute, extension at 72°C fo...
Embodiment 2
[0053] Example 2: cDNA Cloning of IFNα
[0054] Take normal human peripheral blood and add separation lymphocyte stratification solution, separate human leukocytes, culture in DMEM medium containing 5% fetal bovine serum, add Newcastle disease virus and culture at 37°C for 1 hour, centrifuge to discard the supernatant, and add the medium again , 37°C, 5% CO 2 Incubate for 18 hours. Total RNA was isolated with an RNA extraction kit, reverse-transcribed cDNA was obtained with a general-purpose RT-PCR kit (both kits were purchased from Beijing Broadtech Biogene Technology Co., Ltd., and the specific operation was performed according to the instructions), and obtained by PCR. cDNA of IFNα. The primers used are slightly different according to the different IFNα subtypes to be cloned, such as cloning IFNα 2b, IFNα 2a, the primers are available:
[0055] IFNα2b-1: 5'---ATGGATCC TGT GAT CTC CCT CAA ACC CAC AG---3'
[0056] IFNα2b-2: 5'---ATGAATTC TTA TTC CTT ACT CCT TAA TCT TTC TT...
Embodiment 3
[0062] Example 3: cDNA Cloning of IFNβ
[0063] Human amnion cells (Wish cells, provided by the Cell Bank of the Chinese Academy of Sciences) were cultured with 1640 medium containing 10% calf serum until the cells covered the bottom of the bottle, discarded the medium, and added 100 U / ml IFN and 10% calf serum The 1640 medium was continued to be cultured for 16 hours, and the medium was discarded. Add 1640 medium containing 50 μg / ml polyinosinic acid and 10 μg / ml cycloheximide to continue culturing for 4 hours, then add 1 μg / ml actinomycin D for 5 hours. Discard the supernatant, digest with trypsin, collect the cells, use the RNA extraction kit to isolate the total RNA, and use the general RT-PCR kit (both kits were purchased from Beijing Broadtech Biogene Technology Co., Ltd., the specific operation is as follows: Instructions) to obtain reverse-transcribed cDNA, obtain the cDNA of IFNβ by the method of PCR, and its primers can be used:
[0064] IFNβ-1: 5'ATGGATCC ATG AGC ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com