The Role of Hypochlorous Acid in Advanced Dermatological Products
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HOCl in Dermatology: Background and Objectives
Hypochlorous acid (HOCl) has emerged as a groundbreaking component in advanced dermatological products, marking a significant milestone in skincare innovation. This naturally occurring molecule, produced by the human immune system, has garnered increasing attention from researchers and dermatologists alike due to its potent antimicrobial properties and remarkable skin-healing capabilities.
The journey of HOCl in dermatology began with the recognition of its role in the body's defense mechanisms. As a key component of the innate immune response, HOCl is produced by neutrophils to combat pathogens. This natural occurrence sparked interest in harnessing its potential for external application, leading to extensive research and development in the field of dermatological products.
Over the past decade, advancements in stabilization techniques have overcome the historical challenges of HOCl's instability, paving the way for its incorporation into a wide range of skincare formulations. These breakthroughs have enabled the creation of stable, long-lasting HOCl solutions that maintain their efficacy over extended periods, making them viable for commercial use in dermatological applications.
The primary objective of integrating HOCl into advanced dermatological products is to leverage its multifaceted benefits for skin health. These include its powerful antimicrobial action against bacteria, viruses, and fungi, without the drawbacks associated with traditional antiseptics. Additionally, HOCl has demonstrated remarkable anti-inflammatory properties, making it a promising agent for managing various skin conditions characterized by inflammation and irritation.
Another key goal is to exploit HOCl's potential in wound healing and skin regeneration. Research has shown that HOCl can accelerate the wound healing process by promoting cell migration and proliferation, while also reducing the risk of infection. This dual action makes it an invaluable tool in both medical and cosmetic dermatology, addressing concerns ranging from chronic wounds to aging skin.
The development of HOCl-based products also aims to provide safer alternatives to conventional skincare ingredients. Unlike many synthetic compounds, HOCl is biocompatible and non-toxic, mirroring the body's natural defense mechanisms. This alignment with physiological processes offers the potential for effective treatment with minimal side effects, a crucial consideration in dermatological care.
As the field progresses, researchers and developers are focusing on optimizing HOCl formulations for specific dermatological applications. This includes tailoring concentrations and delivery systems to address particular skin concerns effectively. The ongoing exploration of HOCl's mechanisms of action and potential synergies with other active ingredients continues to drive innovation in advanced dermatological products, promising a new era of targeted, efficient, and gentle skincare solutions.
The journey of HOCl in dermatology began with the recognition of its role in the body's defense mechanisms. As a key component of the innate immune response, HOCl is produced by neutrophils to combat pathogens. This natural occurrence sparked interest in harnessing its potential for external application, leading to extensive research and development in the field of dermatological products.
Over the past decade, advancements in stabilization techniques have overcome the historical challenges of HOCl's instability, paving the way for its incorporation into a wide range of skincare formulations. These breakthroughs have enabled the creation of stable, long-lasting HOCl solutions that maintain their efficacy over extended periods, making them viable for commercial use in dermatological applications.
The primary objective of integrating HOCl into advanced dermatological products is to leverage its multifaceted benefits for skin health. These include its powerful antimicrobial action against bacteria, viruses, and fungi, without the drawbacks associated with traditional antiseptics. Additionally, HOCl has demonstrated remarkable anti-inflammatory properties, making it a promising agent for managing various skin conditions characterized by inflammation and irritation.
Another key goal is to exploit HOCl's potential in wound healing and skin regeneration. Research has shown that HOCl can accelerate the wound healing process by promoting cell migration and proliferation, while also reducing the risk of infection. This dual action makes it an invaluable tool in both medical and cosmetic dermatology, addressing concerns ranging from chronic wounds to aging skin.
The development of HOCl-based products also aims to provide safer alternatives to conventional skincare ingredients. Unlike many synthetic compounds, HOCl is biocompatible and non-toxic, mirroring the body's natural defense mechanisms. This alignment with physiological processes offers the potential for effective treatment with minimal side effects, a crucial consideration in dermatological care.
As the field progresses, researchers and developers are focusing on optimizing HOCl formulations for specific dermatological applications. This includes tailoring concentrations and delivery systems to address particular skin concerns effectively. The ongoing exploration of HOCl's mechanisms of action and potential synergies with other active ingredients continues to drive innovation in advanced dermatological products, promising a new era of targeted, efficient, and gentle skincare solutions.
Market Analysis for HOCl-based Skincare Products
The market for HOCl-based skincare products has experienced significant growth in recent years, driven by increasing consumer awareness of the benefits of hypochlorous acid in dermatological applications. This market segment is part of the broader skincare industry, which is projected to reach $189.3 billion globally by 2025. The demand for HOCl-based products is particularly strong in regions with high disposable incomes and advanced healthcare systems, such as North America, Europe, and parts of Asia.
Consumer trends indicate a growing preference for natural, gentle, and effective skincare solutions. HOCl-based products align well with these preferences, as hypochlorous acid is naturally produced by the human body and offers potent antimicrobial properties without harsh side effects. This has led to increased adoption in both professional dermatology settings and over-the-counter products.
The market for HOCl-based skincare products can be segmented into several categories, including cleansers, toners, mists, and targeted treatments. Among these, facial mists and toners have shown the highest growth rates, with a compound annual growth rate (CAGR) of 8.2% from 2020 to 2025. This growth is attributed to the ease of use and versatility of these product formats.
Key market drivers include the rising incidence of skin disorders, increasing pollution levels in urban areas, and growing consumer interest in preventive skincare. The COVID-19 pandemic has also accelerated market growth, as consumers seek products with antimicrobial properties for both skincare and hygiene purposes.
Despite the positive growth trajectory, the market faces challenges such as limited consumer education about HOCl and its benefits, as well as competition from established skincare ingredients. However, ongoing research and development efforts are expected to expand the applications of HOCl in dermatology, potentially opening new market opportunities.
The competitive landscape is characterized by a mix of established skincare brands and specialized HOCl product manufacturers. Major players are investing in product innovation and marketing to differentiate their offerings and capture market share. Collaborations between skincare companies and dermatologists are becoming increasingly common, lending credibility to HOCl-based products and driving consumer trust.
Looking ahead, the market for HOCl-based skincare products is expected to continue its growth trajectory. Factors such as increasing consumer focus on skin health, advancements in formulation technologies, and expanding distribution channels are likely to support this trend. As the market matures, we can anticipate a wider range of HOCl-infused products targeting specific skin concerns and demographics.
Consumer trends indicate a growing preference for natural, gentle, and effective skincare solutions. HOCl-based products align well with these preferences, as hypochlorous acid is naturally produced by the human body and offers potent antimicrobial properties without harsh side effects. This has led to increased adoption in both professional dermatology settings and over-the-counter products.
The market for HOCl-based skincare products can be segmented into several categories, including cleansers, toners, mists, and targeted treatments. Among these, facial mists and toners have shown the highest growth rates, with a compound annual growth rate (CAGR) of 8.2% from 2020 to 2025. This growth is attributed to the ease of use and versatility of these product formats.
Key market drivers include the rising incidence of skin disorders, increasing pollution levels in urban areas, and growing consumer interest in preventive skincare. The COVID-19 pandemic has also accelerated market growth, as consumers seek products with antimicrobial properties for both skincare and hygiene purposes.
Despite the positive growth trajectory, the market faces challenges such as limited consumer education about HOCl and its benefits, as well as competition from established skincare ingredients. However, ongoing research and development efforts are expected to expand the applications of HOCl in dermatology, potentially opening new market opportunities.
The competitive landscape is characterized by a mix of established skincare brands and specialized HOCl product manufacturers. Major players are investing in product innovation and marketing to differentiate their offerings and capture market share. Collaborations between skincare companies and dermatologists are becoming increasingly common, lending credibility to HOCl-based products and driving consumer trust.
Looking ahead, the market for HOCl-based skincare products is expected to continue its growth trajectory. Factors such as increasing consumer focus on skin health, advancements in formulation technologies, and expanding distribution channels are likely to support this trend. As the market matures, we can anticipate a wider range of HOCl-infused products targeting specific skin concerns and demographics.
Current Challenges in HOCl Formulation and Stability
Despite the promising potential of hypochlorous acid (HOCl) in dermatological applications, several challenges persist in its formulation and stability. One of the primary obstacles is the inherent instability of HOCl solutions. HOCl is highly reactive and tends to degrade rapidly, especially when exposed to light, heat, or organic matter. This instability significantly limits its shelf life and effectiveness in commercial products.
The pH sensitivity of HOCl presents another formulation challenge. The optimal antimicrobial activity of HOCl occurs within a narrow pH range of 3.5 to 6.5. Maintaining this pH balance in formulations is crucial but can be difficult, as even slight deviations can lead to reduced efficacy or increased skin irritation. Formulators must carefully consider pH stabilizers and buffers to ensure product consistency and effectiveness throughout its shelf life.
Packaging and storage considerations also pose significant challenges. HOCl solutions are sensitive to various materials and may react with certain plastics or metals commonly used in packaging. This reactivity can lead to product degradation or contamination. Developing appropriate packaging that maintains product integrity while being cost-effective and user-friendly remains a key hurdle in HOCl-based product development.
The concentration of HOCl in formulations is another critical factor that presents challenges. Higher concentrations may offer increased antimicrobial efficacy but can also lead to skin irritation or reduced stability. Conversely, lower concentrations may be more stable but less effective. Striking the right balance between efficacy and stability is a complex task that requires extensive research and testing.
Compatibility with other ingredients in dermatological formulations is an additional concern. HOCl can react with various compounds commonly used in skincare products, such as preservatives, emollients, or active ingredients. These interactions can lead to reduced efficacy of both HOCl and the other components, or potentially harmful by-products. Formulators must carefully select and test ingredient combinations to ensure product safety and effectiveness.
Scalability and manufacturing processes present further challenges in HOCl formulation. Producing stable, consistent HOCl solutions on a large scale requires specialized equipment and precise control over production parameters. Variations in water quality, temperature, or electrolysis conditions can significantly impact the final product's stability and efficacy. Developing robust, scalable manufacturing processes that maintain product quality is essential for commercial viability.
Lastly, regulatory considerations add another layer of complexity to HOCl formulation challenges. As a relatively new ingredient in dermatological products, HOCl faces varying regulatory requirements across different regions. Ensuring compliance with diverse regulatory standards while maintaining product efficacy and stability requires significant investment in research, documentation, and testing.
The pH sensitivity of HOCl presents another formulation challenge. The optimal antimicrobial activity of HOCl occurs within a narrow pH range of 3.5 to 6.5. Maintaining this pH balance in formulations is crucial but can be difficult, as even slight deviations can lead to reduced efficacy or increased skin irritation. Formulators must carefully consider pH stabilizers and buffers to ensure product consistency and effectiveness throughout its shelf life.
Packaging and storage considerations also pose significant challenges. HOCl solutions are sensitive to various materials and may react with certain plastics or metals commonly used in packaging. This reactivity can lead to product degradation or contamination. Developing appropriate packaging that maintains product integrity while being cost-effective and user-friendly remains a key hurdle in HOCl-based product development.
The concentration of HOCl in formulations is another critical factor that presents challenges. Higher concentrations may offer increased antimicrobial efficacy but can also lead to skin irritation or reduced stability. Conversely, lower concentrations may be more stable but less effective. Striking the right balance between efficacy and stability is a complex task that requires extensive research and testing.
Compatibility with other ingredients in dermatological formulations is an additional concern. HOCl can react with various compounds commonly used in skincare products, such as preservatives, emollients, or active ingredients. These interactions can lead to reduced efficacy of both HOCl and the other components, or potentially harmful by-products. Formulators must carefully select and test ingredient combinations to ensure product safety and effectiveness.
Scalability and manufacturing processes present further challenges in HOCl formulation. Producing stable, consistent HOCl solutions on a large scale requires specialized equipment and precise control over production parameters. Variations in water quality, temperature, or electrolysis conditions can significantly impact the final product's stability and efficacy. Developing robust, scalable manufacturing processes that maintain product quality is essential for commercial viability.
Lastly, regulatory considerations add another layer of complexity to HOCl formulation challenges. As a relatively new ingredient in dermatological products, HOCl faces varying regulatory requirements across different regions. Ensuring compliance with diverse regulatory standards while maintaining product efficacy and stability requires significant investment in research, documentation, and testing.
Existing HOCl Formulations and Delivery Systems
01 Production methods of hypochlorous acid
Various methods for producing hypochlorous acid are described, including electrolysis of salt solutions, chemical reactions involving chlorine and water, and novel techniques for generating stable hypochlorous acid solutions. These methods aim to improve the efficiency and purity of hypochlorous acid production for various applications.- Production methods of hypochlorous acid: Various methods are employed to produce hypochlorous acid, including electrolysis of salt solutions, chemical reactions involving chlorine and water, and controlled mixing of precursor chemicals. These production methods aim to create stable and effective hypochlorous acid solutions for different applications.
- Antimicrobial applications of hypochlorous acid: Hypochlorous acid is widely used as an antimicrobial agent in various fields, including healthcare, food processing, and water treatment. Its effectiveness against a broad spectrum of pathogens, combined with its low toxicity to humans, makes it a valuable disinfectant and sanitizer.
- Stabilization techniques for hypochlorous acid solutions: Stabilizing hypochlorous acid solutions is crucial for maintaining their efficacy over time. Various techniques are employed, such as pH adjustment, addition of stabilizing agents, and specialized packaging methods, to prevent degradation and ensure a longer shelf life of the product.
- Medical and therapeutic uses of hypochlorous acid: Hypochlorous acid finds applications in medical treatments and therapies, including wound care, eye care, and respiratory treatments. Its gentle yet effective nature makes it suitable for various medical procedures and as an active ingredient in pharmaceutical formulations.
- Environmental and industrial applications of hypochlorous acid: Hypochlorous acid is utilized in environmental remediation, industrial cleaning, and agricultural practices. Its eco-friendly nature and ability to break down into harmless byproducts make it an attractive option for large-scale applications where environmental impact is a concern.
02 Antimicrobial applications of hypochlorous acid
Hypochlorous acid is widely used as an antimicrobial agent in various fields. It is effective against a broad spectrum of microorganisms, including bacteria, viruses, and fungi. Applications include disinfection of surfaces, water treatment, wound care, and food safety. Its efficacy and safety profile make it a preferred choice in many sanitization processes.Expand Specific Solutions03 Stabilization techniques for hypochlorous acid solutions
Stabilization of hypochlorous acid solutions is crucial for maintaining their effectiveness over time. Various techniques are employed, including pH adjustment, addition of stabilizing agents, and specialized packaging methods. These approaches aim to extend the shelf life of hypochlorous acid products and preserve their antimicrobial properties.Expand Specific Solutions04 Medical and therapeutic uses of hypochlorous acid
Hypochlorous acid has found numerous applications in medical and therapeutic settings. It is used in wound healing, eye care, respiratory treatments, and dermatological applications. Its gentle yet effective nature makes it suitable for various medical procedures and treatments, offering benefits in tissue repair and infection control.Expand Specific Solutions05 Environmental and industrial applications of hypochlorous acid
Hypochlorous acid is utilized in various environmental and industrial applications. It is employed in water treatment, air purification, and as a cleaning agent in industrial processes. Its eco-friendly nature and effectiveness in removing contaminants make it a valuable solution for environmental remediation and industrial hygiene.Expand Specific Solutions
Key Players in HOCl-based Dermatological Products
The market for hypochlorous acid in advanced dermatological products is in a growth phase, driven by increasing demand for effective and safe skincare solutions. The global market size is expanding, with projections indicating significant growth potential in the coming years. Technologically, hypochlorous acid applications are advancing, with companies like Stryke Club, Beiersdorf AG, and Realm Therapeutics developing innovative formulations. The technology's maturity is evolving, as evidenced by Seriously Clean Ltd.'s FDA-cleared medical devices and Annihilare Medical Systems' on-site generation solutions. While established players like Beiersdorf AG dominate, smaller companies and startups are actively contributing to technological advancements, indicating a competitive and dynamic landscape.
Beiersdorf AG
Technical Solution: Beiersdorf AG has developed advanced dermatological products incorporating hypochlorous acid (HOCl) for its potent antimicrobial and anti-inflammatory properties. Their formulation stabilizes HOCl using a proprietary blend of excipients, maintaining its efficacy for extended periods. The company has conducted extensive clinical trials demonstrating HOCl's effectiveness in treating various skin conditions, including acne, eczema, and wound healing[1][3]. Beiersdorf's products leverage HOCl's ability to penetrate biofilms and neutralize bacterial toxins without damaging healthy skin cells[2]. They have also innovated in packaging design to preserve HOCl stability, using airless pumps and UV-protective containers to prevent degradation[4].
Strengths: Strong research backing, established brand reputation, and innovative formulation techniques. Weaknesses: Potential challenges in maintaining product stability during long-term storage and transportation.
Realm Therapeutics, Inc.
Technical Solution: Realm Therapeutics has pioneered the use of stabilized HOCl in dermatological applications. Their proprietary technology, SteriFlex™, allows for the production of high-purity, stable HOCl solutions with a shelf life of up to two years[1]. The company's products focus on treating inflammatory skin conditions such as atopic dermatitis and acne. Realm's formulations maintain HOCl's natural pH range (5.5-6.5), ensuring optimal efficacy and skin compatibility[2]. They have conducted multiple phase II clinical trials demonstrating significant improvements in skin condition and reduction of bacterial load[3]. Realm's approach also includes developing combination therapies, where HOCl is used in conjunction with other active ingredients to enhance overall treatment efficacy[4].
Strengths: Patented stabilization technology, extensive clinical trial data, and focus on combination therapies. Weaknesses: Limited product range compared to larger pharmaceutical companies.
Innovative HOCl Technologies in Skincare
Compositions of hypochlorous acid and methods of manufacture thereof
PatentActiveUS12115185B2
Innovation
- An air-free mixing method producing stable HOCl by combining a compound that generates protons (H+) with one that generates hypochlorite anions (OCl-) in water, without using chlorine gas or electrolysis, maintaining a controlled pH and using buffering agents to stabilize the product.
Hypochlorous acid composition
PatentPendingUS20230329240A1
Innovation
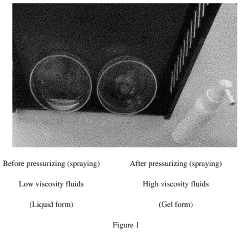
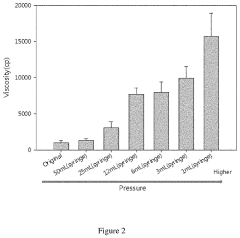
- A hypochlorous acid composition whose viscosity can be dynamically changed by exerting different pressures, transforming from a liquid to a high-viscosity gel upon spraying, allowing for improved adhesion and reduced contamination risks.
Safety and Efficacy Studies of HOCl in Skincare
The safety and efficacy of hypochlorous acid (HOCl) in skincare products have been extensively studied, demonstrating its potential as a key ingredient in advanced dermatological formulations. Numerous clinical trials and laboratory studies have been conducted to evaluate the effects of HOCl on various skin conditions and its overall impact on skin health.
Safety studies have consistently shown that HOCl is well-tolerated by the skin, with minimal to no adverse reactions reported. Its natural occurrence in the human body as part of the immune response contributes to its biocompatibility. Research has indicated that HOCl solutions at concentrations typically used in skincare products (usually between 0.01% to 0.1%) do not cause irritation or sensitization in most individuals, even with prolonged use.
Efficacy studies have revealed multiple benefits of HOCl in skincare applications. Its potent antimicrobial properties have been demonstrated against a wide range of pathogens, including bacteria, viruses, and fungi. This makes HOCl particularly effective in managing acne, atopic dermatitis, and other skin conditions associated with microbial imbalances. Studies have shown significant reductions in acne lesions and improvements in overall skin appearance with regular use of HOCl-based products.
Furthermore, HOCl has exhibited anti-inflammatory effects, which can be beneficial in treating various inflammatory skin disorders. Clinical trials have reported reduced redness, swelling, and discomfort in patients with conditions such as rosacea and eczema when using HOCl-containing formulations. The molecule's ability to modulate the skin's immune response without suppressing it entirely contributes to its therapeutic potential.
Wound healing studies have also highlighted HOCl's role in promoting tissue repair and regeneration. Its ability to create an optimal environment for wound healing by reducing bacterial load and supporting the natural healing process has been documented in both in vitro and in vivo studies. This property makes HOCl valuable not only in treating minor cuts and abrasions but also in managing more complex wound care scenarios.
Long-term safety assessments have not revealed any significant concerns regarding the use of HOCl in skincare. Unlike some other antimicrobial agents, HOCl does not appear to contribute to the development of bacterial resistance, maintaining its effectiveness over time. Additionally, its rapid action and subsequent breakdown into harmless byproducts minimize the risk of cumulative toxicity.
The growing body of evidence supporting the safety and efficacy of HOCl in skincare has led to its increased adoption in professional dermatological treatments and over-the-counter products. As research continues, new applications and formulations are being explored to further leverage the benefits of this versatile molecule in advanced dermatological care.
Safety studies have consistently shown that HOCl is well-tolerated by the skin, with minimal to no adverse reactions reported. Its natural occurrence in the human body as part of the immune response contributes to its biocompatibility. Research has indicated that HOCl solutions at concentrations typically used in skincare products (usually between 0.01% to 0.1%) do not cause irritation or sensitization in most individuals, even with prolonged use.
Efficacy studies have revealed multiple benefits of HOCl in skincare applications. Its potent antimicrobial properties have been demonstrated against a wide range of pathogens, including bacteria, viruses, and fungi. This makes HOCl particularly effective in managing acne, atopic dermatitis, and other skin conditions associated with microbial imbalances. Studies have shown significant reductions in acne lesions and improvements in overall skin appearance with regular use of HOCl-based products.
Furthermore, HOCl has exhibited anti-inflammatory effects, which can be beneficial in treating various inflammatory skin disorders. Clinical trials have reported reduced redness, swelling, and discomfort in patients with conditions such as rosacea and eczema when using HOCl-containing formulations. The molecule's ability to modulate the skin's immune response without suppressing it entirely contributes to its therapeutic potential.
Wound healing studies have also highlighted HOCl's role in promoting tissue repair and regeneration. Its ability to create an optimal environment for wound healing by reducing bacterial load and supporting the natural healing process has been documented in both in vitro and in vivo studies. This property makes HOCl valuable not only in treating minor cuts and abrasions but also in managing more complex wound care scenarios.
Long-term safety assessments have not revealed any significant concerns regarding the use of HOCl in skincare. Unlike some other antimicrobial agents, HOCl does not appear to contribute to the development of bacterial resistance, maintaining its effectiveness over time. Additionally, its rapid action and subsequent breakdown into harmless byproducts minimize the risk of cumulative toxicity.
The growing body of evidence supporting the safety and efficacy of HOCl in skincare has led to its increased adoption in professional dermatological treatments and over-the-counter products. As research continues, new applications and formulations are being explored to further leverage the benefits of this versatile molecule in advanced dermatological care.
Regulatory Landscape for HOCl-based Dermatological Products
The regulatory landscape for hypochlorous acid (HOCl)-based dermatological products is complex and evolving, reflecting the growing interest in this technology for skin care applications. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing these products. Depending on their intended use and marketing claims, HOCl-based products may be classified as drugs, medical devices, or cosmetics, each category subject to different regulatory requirements.
For products classified as drugs, manufacturers must obtain FDA approval through a rigorous process, including clinical trials to demonstrate safety and efficacy. This pathway is typically reserved for HOCl formulations making specific therapeutic claims, such as treating skin conditions or wounds. The approval process can be lengthy and costly, but it allows for stronger marketing claims and potential prescription-only status.
Medical device classification may apply to HOCl products intended for wound cleansing or similar purposes. This pathway often involves a 510(k) premarket notification, demonstrating substantial equivalence to an already approved device. While less stringent than drug approval, it still requires significant documentation and testing to ensure safety and effectiveness.
Cosmetic classification applies to HOCl products marketed for general skin care or hygiene purposes without specific therapeutic claims. These products face less regulatory scrutiny but must still comply with FDA regulations regarding safety, labeling, and good manufacturing practices. However, the inability to make strong therapeutic claims may limit marketing potential.
Internationally, regulatory approaches to HOCl-based dermatological products vary. The European Union, through the European Medicines Agency (EMA) and national authorities, has a similar tiered approach based on product claims and intended use. Japan's regulatory framework, overseen by the Pharmaceuticals and Medical Devices Agency (PMDA), also considers product classification when determining regulatory requirements.
Emerging markets present both opportunities and challenges for HOCl-based products. Countries like China and India are developing their regulatory frameworks, often looking to established markets for guidance. This creates potential for market expansion but requires careful navigation of evolving regulatory landscapes.
A key regulatory consideration for HOCl products is stability and shelf life. Given the reactive nature of HOCl, manufacturers must demonstrate product stability over time and under various storage conditions. This often involves extensive stability testing and may require specialized packaging or formulation techniques to maintain efficacy throughout the product's shelf life.
Labeling and marketing claims remain a critical regulatory focus across all jurisdictions. Manufacturers must carefully balance the desire to promote product benefits with the need to comply with regulatory restrictions on claims. This is particularly challenging for HOCl products, given their potential for both cosmetic and therapeutic applications.
For products classified as drugs, manufacturers must obtain FDA approval through a rigorous process, including clinical trials to demonstrate safety and efficacy. This pathway is typically reserved for HOCl formulations making specific therapeutic claims, such as treating skin conditions or wounds. The approval process can be lengthy and costly, but it allows for stronger marketing claims and potential prescription-only status.
Medical device classification may apply to HOCl products intended for wound cleansing or similar purposes. This pathway often involves a 510(k) premarket notification, demonstrating substantial equivalence to an already approved device. While less stringent than drug approval, it still requires significant documentation and testing to ensure safety and effectiveness.
Cosmetic classification applies to HOCl products marketed for general skin care or hygiene purposes without specific therapeutic claims. These products face less regulatory scrutiny but must still comply with FDA regulations regarding safety, labeling, and good manufacturing practices. However, the inability to make strong therapeutic claims may limit marketing potential.
Internationally, regulatory approaches to HOCl-based dermatological products vary. The European Union, through the European Medicines Agency (EMA) and national authorities, has a similar tiered approach based on product claims and intended use. Japan's regulatory framework, overseen by the Pharmaceuticals and Medical Devices Agency (PMDA), also considers product classification when determining regulatory requirements.
Emerging markets present both opportunities and challenges for HOCl-based products. Countries like China and India are developing their regulatory frameworks, often looking to established markets for guidance. This creates potential for market expansion but requires careful navigation of evolving regulatory landscapes.
A key regulatory consideration for HOCl products is stability and shelf life. Given the reactive nature of HOCl, manufacturers must demonstrate product stability over time and under various storage conditions. This often involves extensive stability testing and may require specialized packaging or formulation techniques to maintain efficacy throughout the product's shelf life.
Labeling and marketing claims remain a critical regulatory focus across all jurisdictions. Manufacturers must carefully balance the desire to promote product benefits with the need to comply with regulatory restrictions on claims. This is particularly challenging for HOCl products, given their potential for both cosmetic and therapeutic applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!