Kevlar Applications in the Development of Advanced Orthotics
JUL 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Kevlar Orthotics Evolution
The evolution of Kevlar applications in orthotics has been marked by significant advancements in material science and biomechanical engineering. Initially developed by DuPont in the 1960s, Kevlar's exceptional strength-to-weight ratio and durability quickly found applications beyond its original use in tire reinforcement.
In the 1980s, researchers began exploring Kevlar's potential in medical devices, particularly orthotics. The first Kevlar-reinforced orthoses were primarily used in lower limb braces, offering improved support and reduced weight compared to traditional metal and plastic designs. These early applications demonstrated Kevlar's ability to enhance patient comfort while maintaining structural integrity.
The 1990s saw a surge in research focused on optimizing Kevlar composites for orthotic applications. Scientists experimented with various fiber orientations and resin systems to create materials that could better mimic the biomechanical properties of human tissues. This period also marked the introduction of Kevlar-based ankle-foot orthoses (AFOs), which provided superior energy return and reduced fatigue for patients with neuromuscular disorders.
By the early 2000s, advanced manufacturing techniques, such as 3D printing and computer-aided design, began to revolutionize orthotic production. These technologies allowed for the creation of highly customized Kevlar-reinforced devices tailored to individual patient needs. Researchers also started investigating hybrid materials, combining Kevlar with other high-performance fibers like carbon and glass to further enhance orthotic performance.
The past decade has witnessed a shift towards smart orthotics incorporating Kevlar. Researchers have developed prototypes that integrate sensors and actuators within Kevlar-based structures, enabling real-time monitoring and adjustment of orthotic support. This fusion of material science and electronics has opened new possibilities for adaptive and responsive orthotic devices.
Recent advancements have focused on improving the biocompatibility and sustainability of Kevlar-based orthotics. Scientists are exploring bio-based alternatives and surface treatments to enhance the material's interaction with human tissues. Additionally, efforts are being made to develop recycling processes for Kevlar composites, addressing environmental concerns associated with orthotic disposal.
Looking ahead, the evolution of Kevlar in orthotics is likely to continue along several trajectories. These include the development of even lighter and stronger Kevlar variants, further integration with smart technologies, and exploration of biodegradable Kevlar-like materials. As research progresses, Kevlar-based orthotics are poised to play an increasingly crucial role in improving mobility and quality of life for individuals with musculoskeletal and neuromuscular conditions.
In the 1980s, researchers began exploring Kevlar's potential in medical devices, particularly orthotics. The first Kevlar-reinforced orthoses were primarily used in lower limb braces, offering improved support and reduced weight compared to traditional metal and plastic designs. These early applications demonstrated Kevlar's ability to enhance patient comfort while maintaining structural integrity.
The 1990s saw a surge in research focused on optimizing Kevlar composites for orthotic applications. Scientists experimented with various fiber orientations and resin systems to create materials that could better mimic the biomechanical properties of human tissues. This period also marked the introduction of Kevlar-based ankle-foot orthoses (AFOs), which provided superior energy return and reduced fatigue for patients with neuromuscular disorders.
By the early 2000s, advanced manufacturing techniques, such as 3D printing and computer-aided design, began to revolutionize orthotic production. These technologies allowed for the creation of highly customized Kevlar-reinforced devices tailored to individual patient needs. Researchers also started investigating hybrid materials, combining Kevlar with other high-performance fibers like carbon and glass to further enhance orthotic performance.
The past decade has witnessed a shift towards smart orthotics incorporating Kevlar. Researchers have developed prototypes that integrate sensors and actuators within Kevlar-based structures, enabling real-time monitoring and adjustment of orthotic support. This fusion of material science and electronics has opened new possibilities for adaptive and responsive orthotic devices.
Recent advancements have focused on improving the biocompatibility and sustainability of Kevlar-based orthotics. Scientists are exploring bio-based alternatives and surface treatments to enhance the material's interaction with human tissues. Additionally, efforts are being made to develop recycling processes for Kevlar composites, addressing environmental concerns associated with orthotic disposal.
Looking ahead, the evolution of Kevlar in orthotics is likely to continue along several trajectories. These include the development of even lighter and stronger Kevlar variants, further integration with smart technologies, and exploration of biodegradable Kevlar-like materials. As research progresses, Kevlar-based orthotics are poised to play an increasingly crucial role in improving mobility and quality of life for individuals with musculoskeletal and neuromuscular conditions.
Orthopedic Market Analysis
The orthopedic market has experienced significant growth in recent years, driven by an aging population, increasing prevalence of musculoskeletal disorders, and advancements in medical technology. The global orthopedic devices market was valued at approximately $53 billion in 2020 and is projected to reach $68 billion by 2026, growing at a CAGR of around 4.3% during the forecast period.
Within this market, the orthotics segment plays a crucial role, addressing various musculoskeletal conditions and improving patient mobility. The global orthotics market size was estimated at $3.1 billion in 2020 and is expected to expand at a CAGR of 6.2% from 2021 to 2028. This growth is attributed to the rising incidence of sports injuries, increasing awareness about the benefits of orthotic devices, and technological advancements in materials and manufacturing processes.
The demand for advanced orthotics is particularly strong in developed regions such as North America and Europe, where healthcare infrastructure is well-established and there is a higher adoption rate of innovative medical technologies. However, emerging economies in Asia-Pacific and Latin America are also witnessing rapid growth in the orthotics market due to improving healthcare access and rising disposable incomes.
Key factors driving the orthopedic market include the growing prevalence of osteoarthritis, rheumatoid arthritis, and other degenerative joint diseases. Additionally, the rise in sports-related injuries and the increasing focus on preventive healthcare are contributing to market expansion. The COVID-19 pandemic has also accelerated the adoption of telemedicine and remote patient monitoring in orthopedic care, creating new opportunities for digital health solutions in this sector.
The introduction of advanced materials like Kevlar in orthotic devices represents a significant technological advancement in the field. Kevlar, known for its high strength-to-weight ratio and durability, offers potential benefits in terms of improved performance, reduced weight, and enhanced comfort for patients. This innovation aligns with the broader trend of material science advancements in the orthopedic industry, which aims to develop more effective, lightweight, and customizable orthotic solutions.
Market segmentation reveals that lower limb orthotics dominate the market share, followed by upper limb orthotics and spinal orthotics. The increasing prevalence of foot and ankle disorders, coupled with the rising demand for sports-related orthotics, is driving growth in the lower limb segment. Furthermore, the integration of smart technologies and 3D printing in orthotic manufacturing is expected to revolutionize the market, enabling more personalized and efficient production processes.
In conclusion, the orthopedic market, particularly the orthotics segment, presents significant growth opportunities driven by demographic trends, technological advancements, and increasing healthcare awareness. The application of advanced materials like Kevlar in orthotic development aligns with market demands for improved performance and patient comfort, positioning it as a promising area for innovation and market expansion.
Within this market, the orthotics segment plays a crucial role, addressing various musculoskeletal conditions and improving patient mobility. The global orthotics market size was estimated at $3.1 billion in 2020 and is expected to expand at a CAGR of 6.2% from 2021 to 2028. This growth is attributed to the rising incidence of sports injuries, increasing awareness about the benefits of orthotic devices, and technological advancements in materials and manufacturing processes.
The demand for advanced orthotics is particularly strong in developed regions such as North America and Europe, where healthcare infrastructure is well-established and there is a higher adoption rate of innovative medical technologies. However, emerging economies in Asia-Pacific and Latin America are also witnessing rapid growth in the orthotics market due to improving healthcare access and rising disposable incomes.
Key factors driving the orthopedic market include the growing prevalence of osteoarthritis, rheumatoid arthritis, and other degenerative joint diseases. Additionally, the rise in sports-related injuries and the increasing focus on preventive healthcare are contributing to market expansion. The COVID-19 pandemic has also accelerated the adoption of telemedicine and remote patient monitoring in orthopedic care, creating new opportunities for digital health solutions in this sector.
The introduction of advanced materials like Kevlar in orthotic devices represents a significant technological advancement in the field. Kevlar, known for its high strength-to-weight ratio and durability, offers potential benefits in terms of improved performance, reduced weight, and enhanced comfort for patients. This innovation aligns with the broader trend of material science advancements in the orthopedic industry, which aims to develop more effective, lightweight, and customizable orthotic solutions.
Market segmentation reveals that lower limb orthotics dominate the market share, followed by upper limb orthotics and spinal orthotics. The increasing prevalence of foot and ankle disorders, coupled with the rising demand for sports-related orthotics, is driving growth in the lower limb segment. Furthermore, the integration of smart technologies and 3D printing in orthotic manufacturing is expected to revolutionize the market, enabling more personalized and efficient production processes.
In conclusion, the orthopedic market, particularly the orthotics segment, presents significant growth opportunities driven by demographic trends, technological advancements, and increasing healthcare awareness. The application of advanced materials like Kevlar in orthotic development aligns with market demands for improved performance and patient comfort, positioning it as a promising area for innovation and market expansion.
Kevlar Tech Challenges
Despite the remarkable properties of Kevlar in orthotics development, several technical challenges persist in its application. One of the primary obstacles is the difficulty in achieving optimal fiber orientation and distribution within the orthotic structure. The anisotropic nature of Kevlar fibers requires precise alignment to maximize strength and stiffness in specific directions, which can be challenging to maintain consistently during the manufacturing process.
Another significant challenge lies in the bonding of Kevlar to other materials commonly used in orthotics, such as thermoplastics or carbon fiber composites. The chemical inertness of Kevlar, while beneficial for its durability, makes it resistant to adhesion. This necessitates the development of specialized bonding techniques or surface treatments to ensure strong, lasting interfaces between Kevlar and other components of the orthotic device.
The integration of Kevlar into complex, three-dimensional orthotic shapes presents additional difficulties. Traditional manufacturing methods may struggle to conform Kevlar sheets or fibers to intricate contours without compromising the material's structural integrity or creating weak points. This challenge is particularly evident in custom-fitted orthotics that require precise anatomical conformity.
Durability under repeated stress and environmental factors is another area of concern. While Kevlar exhibits excellent tensile strength, its performance under cyclic loading, particularly in areas of flexion or articulation in orthotics, requires further investigation. Additionally, long-term exposure to moisture, UV radiation, and temperature fluctuations may affect the material's properties over time, necessitating the development of protective coatings or treatments.
The high cost of Kevlar compared to conventional orthotic materials poses an economic challenge to its widespread adoption. Balancing the superior mechanical properties of Kevlar with cost-effectiveness remains a key consideration for manufacturers and healthcare providers alike. This economic factor drives the need for more efficient manufacturing processes and innovative design approaches that optimize Kevlar usage.
Lastly, the recyclability and end-of-life management of Kevlar-reinforced orthotics present environmental challenges. The durability that makes Kevlar desirable also makes it resistant to biodegradation. Developing sustainable disposal or recycling methods for Kevlar-containing orthotics is crucial for minimizing environmental impact and aligning with circular economy principles in healthcare product design.
Addressing these technical challenges requires interdisciplinary collaboration among materials scientists, biomedical engineers, and orthotic specialists. Ongoing research focuses on advanced manufacturing techniques, such as 3D printing with Kevlar-reinforced filaments, to overcome some of these obstacles. Additionally, the development of hybrid materials that combine Kevlar with other high-performance fibers or polymers shows promise in mitigating some of the material's limitations while leveraging its strengths.
Another significant challenge lies in the bonding of Kevlar to other materials commonly used in orthotics, such as thermoplastics or carbon fiber composites. The chemical inertness of Kevlar, while beneficial for its durability, makes it resistant to adhesion. This necessitates the development of specialized bonding techniques or surface treatments to ensure strong, lasting interfaces between Kevlar and other components of the orthotic device.
The integration of Kevlar into complex, three-dimensional orthotic shapes presents additional difficulties. Traditional manufacturing methods may struggle to conform Kevlar sheets or fibers to intricate contours without compromising the material's structural integrity or creating weak points. This challenge is particularly evident in custom-fitted orthotics that require precise anatomical conformity.
Durability under repeated stress and environmental factors is another area of concern. While Kevlar exhibits excellent tensile strength, its performance under cyclic loading, particularly in areas of flexion or articulation in orthotics, requires further investigation. Additionally, long-term exposure to moisture, UV radiation, and temperature fluctuations may affect the material's properties over time, necessitating the development of protective coatings or treatments.
The high cost of Kevlar compared to conventional orthotic materials poses an economic challenge to its widespread adoption. Balancing the superior mechanical properties of Kevlar with cost-effectiveness remains a key consideration for manufacturers and healthcare providers alike. This economic factor drives the need for more efficient manufacturing processes and innovative design approaches that optimize Kevlar usage.
Lastly, the recyclability and end-of-life management of Kevlar-reinforced orthotics present environmental challenges. The durability that makes Kevlar desirable also makes it resistant to biodegradation. Developing sustainable disposal or recycling methods for Kevlar-containing orthotics is crucial for minimizing environmental impact and aligning with circular economy principles in healthcare product design.
Addressing these technical challenges requires interdisciplinary collaboration among materials scientists, biomedical engineers, and orthotic specialists. Ongoing research focuses on advanced manufacturing techniques, such as 3D printing with Kevlar-reinforced filaments, to overcome some of these obstacles. Additionally, the development of hybrid materials that combine Kevlar with other high-performance fibers or polymers shows promise in mitigating some of the material's limitations while leveraging its strengths.
Current Kevlar Solutions
01 Kevlar-reinforced composite materials
Kevlar fibers are used to reinforce various composite materials, enhancing their strength, durability, and impact resistance. These composites find applications in aerospace, automotive, and protective equipment industries.- Kevlar-reinforced composite materials: Kevlar fibers are used to reinforce various composite materials, enhancing their strength, durability, and impact resistance. These composites find applications in aerospace, automotive, and protective equipment industries.
- Kevlar in protective gear and clothing: Kevlar is extensively used in the manufacture of protective gear and clothing, including bulletproof vests, helmets, and cut-resistant gloves. Its high tensile strength and lightweight properties make it ideal for personal protection equipment.
- Kevlar-based fire-resistant materials: Kevlar is utilized in the development of fire-resistant materials and fabrics. These materials are used in firefighting equipment, industrial safety gear, and building materials to enhance fire protection and safety.
- Kevlar in automotive applications: Kevlar is employed in various automotive components to reduce weight while maintaining strength. It is used in tire reinforcement, brake pads, and body panels to improve vehicle performance and fuel efficiency.
- Kevlar in aerospace and marine industries: Kevlar finds applications in aerospace and marine industries for its high strength-to-weight ratio. It is used in aircraft components, spacecraft heat shields, and boat hulls to enhance performance and durability in extreme conditions.
02 Kevlar in protective gear and clothing
Kevlar is widely used in the manufacture of protective gear and clothing, including bulletproof vests, helmets, and cut-resistant gloves. Its high tensile strength and lightweight properties make it ideal for personal protection equipment.Expand Specific Solutions03 Kevlar-based fire-resistant materials
Kevlar is utilized in the development of fire-resistant materials and fabrics. These materials are used in firefighting equipment, industrial safety gear, and other applications requiring heat and flame resistance.Expand Specific Solutions04 Kevlar in automotive applications
Kevlar is employed in various automotive components to reduce weight while maintaining strength. It is used in tire reinforcement, brake pads, and other parts to improve vehicle performance and fuel efficiency.Expand Specific Solutions05 Kevlar in aerospace and marine applications
Kevlar is utilized in aerospace and marine industries for its high strength-to-weight ratio. It is used in aircraft components, boat hulls, and other structures to enhance durability and reduce overall weight.Expand Specific Solutions
Key Orthotic Manufacturers
The application of Kevlar in advanced orthotics represents a dynamic and evolving field within the medical technology sector. The market is in a growth phase, driven by increasing demand for lightweight, durable, and high-performance orthotic solutions. While the exact market size is not specified, the presence of major players like Stryker Corp. and Warsaw Orthopedic (part of Medtronic) indicates significant commercial potential. The technology's maturity is advancing, with research institutions such as Changchun University of Technology and Politecnico di Torino contributing to its development. Companies like Wright Medical Technology and Align Technology are likely exploring innovative applications, suggesting a competitive landscape where both established medical device manufacturers and specialized orthotics firms are vying for market share.
Stryker Corp.
Technical Solution: Stryker Corp. has developed advanced orthotics using Kevlar, focusing on lightweight and durable designs. Their approach involves integrating Kevlar fibers into composite materials to create orthotic devices with enhanced strength-to-weight ratios. The company has implemented a proprietary manufacturing process that allows for precise layering of Kevlar-reinforced materials, resulting in orthotics that provide superior support while maintaining flexibility[1]. Stryker's Kevlar-enhanced orthotics have shown particular promise in sports medicine applications, where they offer improved impact resistance and energy return compared to traditional materials[3].
Strengths: Superior strength-to-weight ratio, excellent durability, and enhanced impact resistance. Weaknesses: Potentially higher production costs and limited customization options compared to some traditional materials.
Warsaw Orthopedic, Inc.
Technical Solution: Warsaw Orthopedic has pioneered the use of Kevlar in spinal orthotics, developing a range of products that leverage the material's unique properties. Their innovative approach involves combining Kevlar fibers with biocompatible polymers to create spinal braces and supports that offer exceptional stability and comfort. The company has patented a process for weaving Kevlar into three-dimensional structures that conform to the spine's natural curvature, providing targeted support where it's most needed[2]. Warsaw's Kevlar-based spinal orthotics have demonstrated significant improvements in patient outcomes, particularly in cases of scoliosis and post-operative recovery[4].
Strengths: Excellent conformability to spinal anatomy, high tensile strength, and improved patient outcomes. Weaknesses: Limited application outside of spinal orthotics and potential for higher costs compared to traditional materials.
Kevlar Orthotics Patents
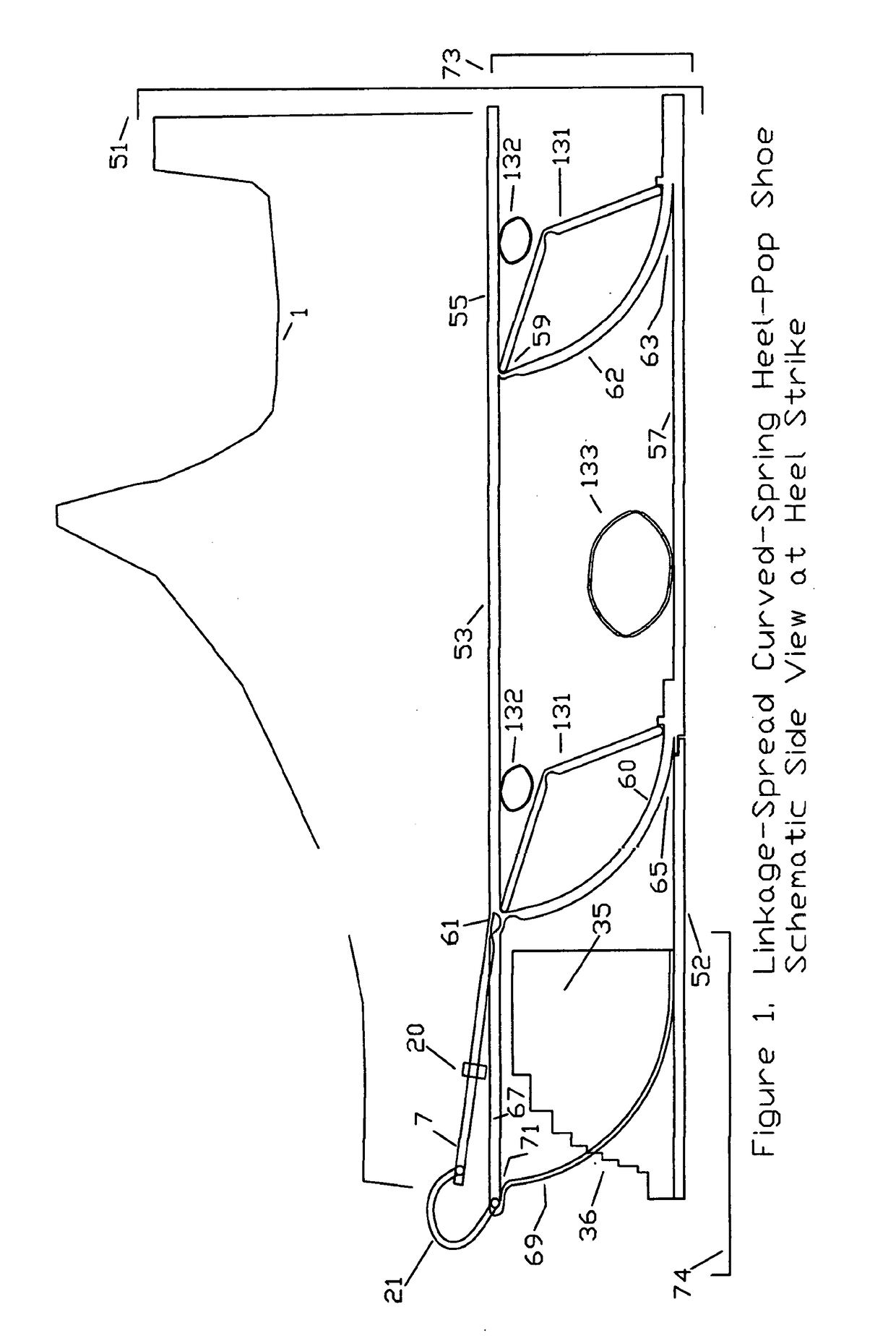
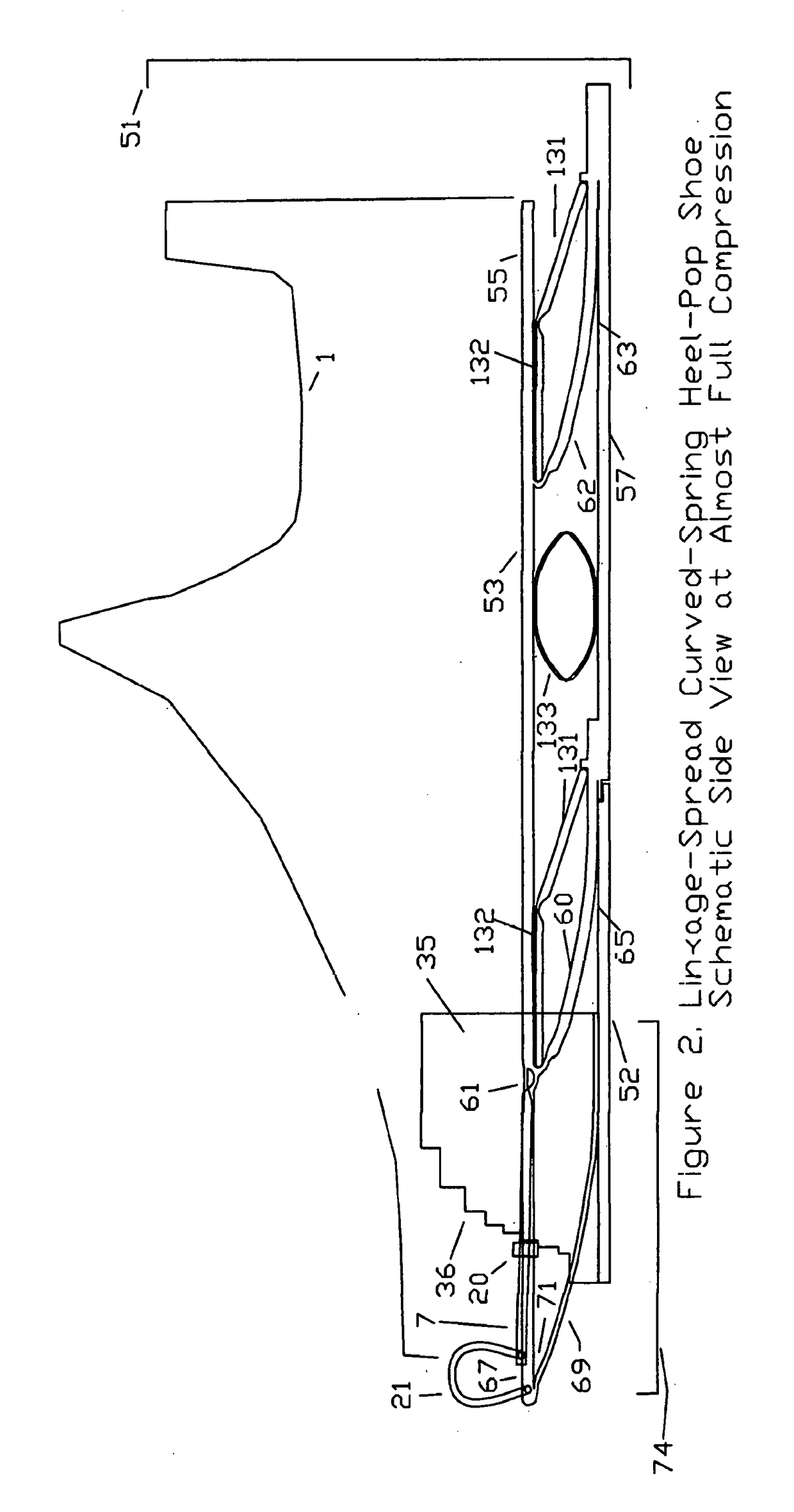
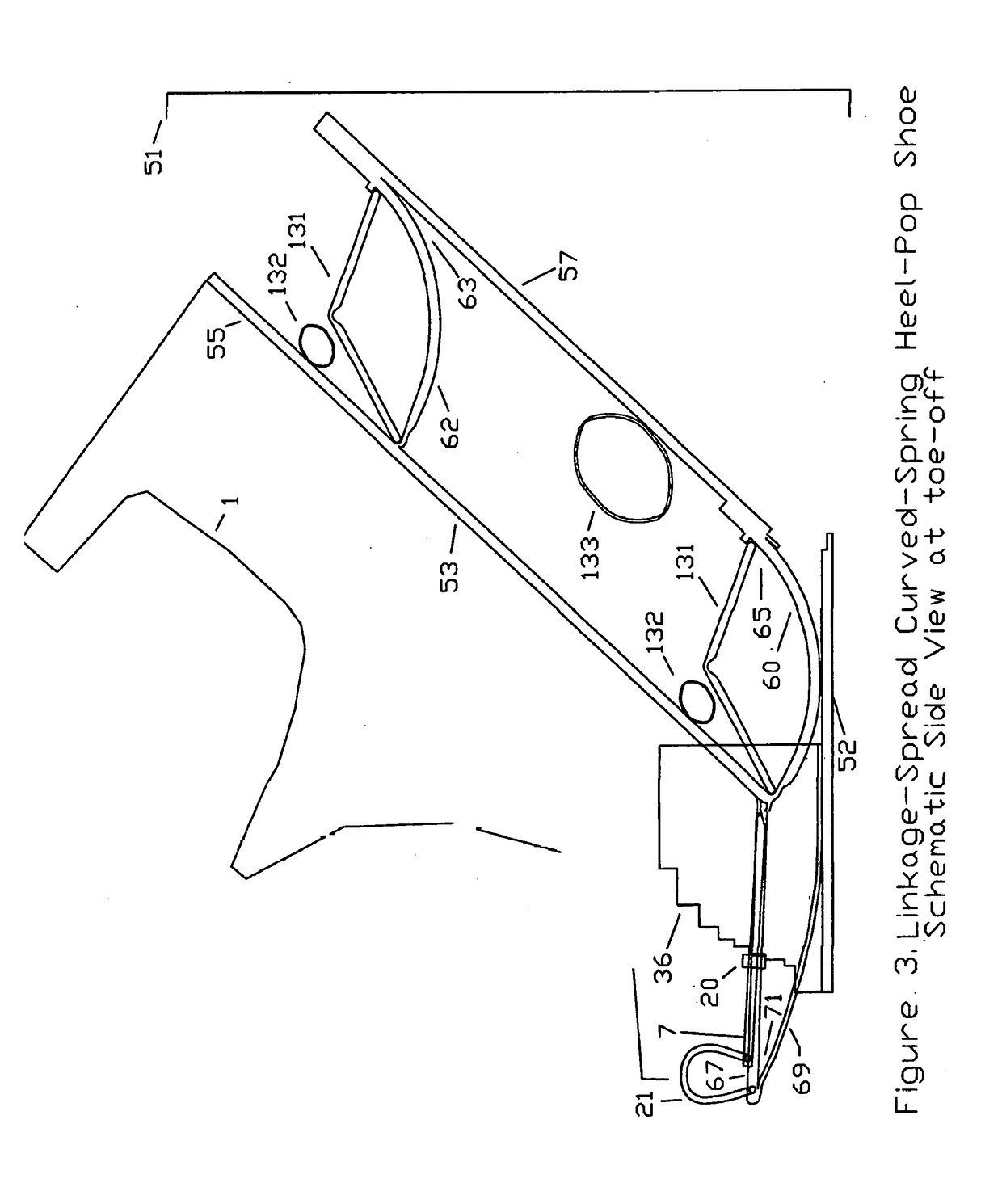
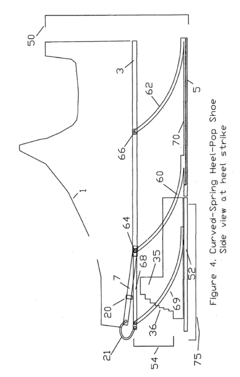
Substantial energy return shoe with optimal low-impact springs, tuned gear change, and smart knee brace
PatentInactiveUS20180220738A1
Innovation
- The design incorporates enhanced heel-lift mechanisms with anti-toe-sink mechanisms and automatic gear changers, utilizing optimized low-impact springs and linkage systems to ensure full sole compression and efficient energy storage and release, reducing the maximum impact force and increasing energy return.
Kevlar and nanohydroxyapatite based bone replacement to counter knee jerk
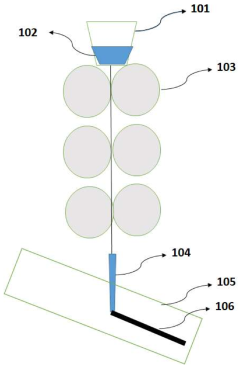
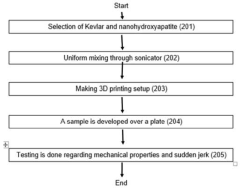
PatentPendingIN202111041724A
Innovation
- A nanocomposite material composed of Kevlar fiber and nanohydroxyapatite is developed using additive manufacturing techniques like 3D printing, which enhances mechanical properties and biocompatibility, and is tested for impact resistance through a specific manufacturing and testing process.
Biomechanical Testing
Biomechanical testing plays a crucial role in evaluating the effectiveness of Kevlar applications in advanced orthotics development. This testing methodology provides quantitative data on the performance of Kevlar-reinforced orthotic devices under various loading conditions and movement patterns.
One of the primary focuses of biomechanical testing for Kevlar-enhanced orthotics is the assessment of strength-to-weight ratio. Kevlar's exceptional tensile strength combined with its lightweight nature makes it an ideal material for orthotic devices. Through controlled laboratory experiments, researchers can measure the load-bearing capacity of Kevlar-reinforced orthotics and compare it to traditional materials such as carbon fiber or thermoplastics.
Fatigue testing is another critical aspect of biomechanical evaluation for Kevlar orthotics. This involves subjecting the orthotic devices to repetitive loading cycles that simulate real-world usage conditions. By analyzing the material's response to cyclic stress, researchers can determine the long-term durability and reliability of Kevlar-enhanced orthotics, ensuring they maintain their structural integrity over extended periods of use.
Impact resistance testing is particularly relevant for orthotics designed for high-impact activities or sports applications. Kevlar's ability to absorb and dissipate energy makes it an excellent choice for such scenarios. Biomechanical tests involving drop impacts or simulated collisions can provide valuable data on the protective capabilities of Kevlar-reinforced orthotics, potentially leading to improved safety features in advanced orthotic designs.
Gait analysis forms an integral part of biomechanical testing for Kevlar orthotics. By using motion capture systems and force plates, researchers can assess how Kevlar-enhanced orthotics affect an individual's walking or running patterns. This analysis helps in understanding the device's influence on joint kinematics, muscle activation, and overall movement efficiency, which are crucial factors in orthotic design optimization.
Pressure distribution mapping is another valuable tool in biomechanical testing. By using pressure-sensitive mats or in-shoe pressure measurement systems, researchers can evaluate how Kevlar orthotics redistribute pressure across the foot or affected body part. This information is vital for designing orthotics that provide optimal support and comfort while preventing pressure-related complications.
Thermal testing is also considered in biomechanical evaluations, as the comfort and breathability of orthotics are essential for user compliance. Although Kevlar is known for its heat resistance, it's crucial to assess how it performs in combination with other materials used in orthotic construction. Temperature and humidity sensors can be employed to measure heat dissipation and moisture management properties of Kevlar-enhanced orthotics during simulated use conditions.
One of the primary focuses of biomechanical testing for Kevlar-enhanced orthotics is the assessment of strength-to-weight ratio. Kevlar's exceptional tensile strength combined with its lightweight nature makes it an ideal material for orthotic devices. Through controlled laboratory experiments, researchers can measure the load-bearing capacity of Kevlar-reinforced orthotics and compare it to traditional materials such as carbon fiber or thermoplastics.
Fatigue testing is another critical aspect of biomechanical evaluation for Kevlar orthotics. This involves subjecting the orthotic devices to repetitive loading cycles that simulate real-world usage conditions. By analyzing the material's response to cyclic stress, researchers can determine the long-term durability and reliability of Kevlar-enhanced orthotics, ensuring they maintain their structural integrity over extended periods of use.
Impact resistance testing is particularly relevant for orthotics designed for high-impact activities or sports applications. Kevlar's ability to absorb and dissipate energy makes it an excellent choice for such scenarios. Biomechanical tests involving drop impacts or simulated collisions can provide valuable data on the protective capabilities of Kevlar-reinforced orthotics, potentially leading to improved safety features in advanced orthotic designs.
Gait analysis forms an integral part of biomechanical testing for Kevlar orthotics. By using motion capture systems and force plates, researchers can assess how Kevlar-enhanced orthotics affect an individual's walking or running patterns. This analysis helps in understanding the device's influence on joint kinematics, muscle activation, and overall movement efficiency, which are crucial factors in orthotic design optimization.
Pressure distribution mapping is another valuable tool in biomechanical testing. By using pressure-sensitive mats or in-shoe pressure measurement systems, researchers can evaluate how Kevlar orthotics redistribute pressure across the foot or affected body part. This information is vital for designing orthotics that provide optimal support and comfort while preventing pressure-related complications.
Thermal testing is also considered in biomechanical evaluations, as the comfort and breathability of orthotics are essential for user compliance. Although Kevlar is known for its heat resistance, it's crucial to assess how it performs in combination with other materials used in orthotic construction. Temperature and humidity sensors can be employed to measure heat dissipation and moisture management properties of Kevlar-enhanced orthotics during simulated use conditions.
Regulatory Compliance
The development and application of Kevlar in advanced orthotics must adhere to stringent regulatory standards to ensure patient safety and product efficacy. In the United States, the Food and Drug Administration (FDA) classifies orthotics as Class I or Class II medical devices, depending on their intended use and level of risk. Kevlar-based orthotics typically fall under Class II, requiring a 510(k) premarket notification submission to demonstrate substantial equivalence to a legally marketed predicate device.
Manufacturers must comply with the FDA's Quality System Regulation (QSR), which includes requirements for design controls, manufacturing processes, and post-market surveillance. This involves implementing a robust quality management system, conducting risk assessments, and maintaining detailed documentation throughout the product lifecycle. Additionally, Good Manufacturing Practices (GMP) must be followed to ensure consistent product quality and safety.
In the European Union, Kevlar-based orthotics are regulated under the Medical Device Regulation (MDR). Manufacturers must obtain CE marking by demonstrating compliance with the MDR's essential requirements, which include safety, performance, and risk management. This process involves conducting clinical evaluations, preparing technical documentation, and implementing a post-market surveillance system.
International standards such as ISO 13485 for medical device quality management systems and ISO 14971 for risk management in medical devices play a crucial role in regulatory compliance. Adherence to these standards helps manufacturers meet global regulatory requirements and facilitates market access across different regions.
Material safety is a key consideration in regulatory compliance for Kevlar-based orthotics. Manufacturers must provide evidence of biocompatibility testing in accordance with ISO 10993 standards to demonstrate that the materials used are safe for prolonged skin contact. This includes evaluating potential cytotoxicity, sensitization, and irritation effects.
Labeling and packaging regulations are also critical aspects of compliance. Clear, accurate, and comprehensive labeling must be provided, including information on intended use, contraindications, and proper care instructions. In the US, this is governed by the FDA's labeling regulations, while in the EU, it falls under the MDR's requirements for information supplied with the device.
Post-market surveillance and vigilance are ongoing regulatory obligations for manufacturers of Kevlar-based orthotics. This involves monitoring the performance and safety of devices in real-world use, collecting and analyzing customer feedback, and reporting adverse events to regulatory authorities when necessary. Manufacturers must have systems in place to track and trend this information, enabling continuous improvement and timely response to any emerging safety concerns.
Manufacturers must comply with the FDA's Quality System Regulation (QSR), which includes requirements for design controls, manufacturing processes, and post-market surveillance. This involves implementing a robust quality management system, conducting risk assessments, and maintaining detailed documentation throughout the product lifecycle. Additionally, Good Manufacturing Practices (GMP) must be followed to ensure consistent product quality and safety.
In the European Union, Kevlar-based orthotics are regulated under the Medical Device Regulation (MDR). Manufacturers must obtain CE marking by demonstrating compliance with the MDR's essential requirements, which include safety, performance, and risk management. This process involves conducting clinical evaluations, preparing technical documentation, and implementing a post-market surveillance system.
International standards such as ISO 13485 for medical device quality management systems and ISO 14971 for risk management in medical devices play a crucial role in regulatory compliance. Adherence to these standards helps manufacturers meet global regulatory requirements and facilitates market access across different regions.
Material safety is a key consideration in regulatory compliance for Kevlar-based orthotics. Manufacturers must provide evidence of biocompatibility testing in accordance with ISO 10993 standards to demonstrate that the materials used are safe for prolonged skin contact. This includes evaluating potential cytotoxicity, sensitization, and irritation effects.
Labeling and packaging regulations are also critical aspects of compliance. Clear, accurate, and comprehensive labeling must be provided, including information on intended use, contraindications, and proper care instructions. In the US, this is governed by the FDA's labeling regulations, while in the EU, it falls under the MDR's requirements for information supplied with the device.
Post-market surveillance and vigilance are ongoing regulatory obligations for manufacturers of Kevlar-based orthotics. This involves monitoring the performance and safety of devices in real-world use, collecting and analyzing customer feedback, and reporting adverse events to regulatory authorities when necessary. Manufacturers must have systems in place to track and trend this information, enabling continuous improvement and timely response to any emerging safety concerns.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!