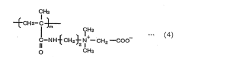
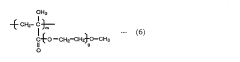
Isocyanate Use in Medical Devices: Recent Innovations
JUL 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isocyanate Evolution in Medical Devices
Isocyanates have played a significant role in the development of medical devices over the past few decades. Initially introduced in the 1930s, these versatile compounds found their way into medical applications in the 1960s, primarily as components in polyurethane-based materials. The evolution of isocyanates in medical devices has been marked by continuous improvements in biocompatibility, mechanical properties, and manufacturing processes.
In the early stages, isocyanates were primarily used in the production of rigid and flexible foams for medical cushioning and support devices. As research progressed, their application expanded to include the development of catheters, wound dressings, and implantable devices. The 1980s saw a surge in the use of isocyanate-based polyurethanes for cardiovascular applications, such as artificial heart valves and vascular grafts, due to their excellent mechanical properties and blood compatibility.
The 1990s brought increased focus on the potential health risks associated with isocyanate exposure during manufacturing processes. This led to the development of safer handling procedures and the exploration of alternative chemistries. Concurrently, advancements in polymer science enabled the creation of more sophisticated isocyanate-based materials with enhanced biocompatibility and controlled degradation profiles.
The turn of the millennium marked a shift towards the use of aliphatic isocyanates, which offered improved stability and resistance to yellowing compared to their aromatic counterparts. This innovation expanded the use of isocyanates in long-term implantable devices and transparent medical components. Additionally, the introduction of water-based polyurethane dispersions reduced the environmental impact and improved worker safety in medical device manufacturing.
Recent years have seen the emergence of novel isocyanate chemistries tailored for specific medical applications. These include the development of shape-memory polyurethanes for minimally invasive surgical devices and the incorporation of isocyanate-based materials in drug delivery systems. The advent of 3D printing technologies has also opened new avenues for the use of isocyanate-based resins in the production of customized medical devices and implants.
Looking ahead, the evolution of isocyanates in medical devices is likely to focus on further enhancing biocompatibility, developing smart materials with responsive properties, and exploring sustainable production methods. The integration of nanotechnology and the development of hybrid materials combining isocyanates with other advanced polymers are expected to drive innovation in this field, potentially revolutionizing the design and functionality of future medical devices.
In the early stages, isocyanates were primarily used in the production of rigid and flexible foams for medical cushioning and support devices. As research progressed, their application expanded to include the development of catheters, wound dressings, and implantable devices. The 1980s saw a surge in the use of isocyanate-based polyurethanes for cardiovascular applications, such as artificial heart valves and vascular grafts, due to their excellent mechanical properties and blood compatibility.
The 1990s brought increased focus on the potential health risks associated with isocyanate exposure during manufacturing processes. This led to the development of safer handling procedures and the exploration of alternative chemistries. Concurrently, advancements in polymer science enabled the creation of more sophisticated isocyanate-based materials with enhanced biocompatibility and controlled degradation profiles.
The turn of the millennium marked a shift towards the use of aliphatic isocyanates, which offered improved stability and resistance to yellowing compared to their aromatic counterparts. This innovation expanded the use of isocyanates in long-term implantable devices and transparent medical components. Additionally, the introduction of water-based polyurethane dispersions reduced the environmental impact and improved worker safety in medical device manufacturing.
Recent years have seen the emergence of novel isocyanate chemistries tailored for specific medical applications. These include the development of shape-memory polyurethanes for minimally invasive surgical devices and the incorporation of isocyanate-based materials in drug delivery systems. The advent of 3D printing technologies has also opened new avenues for the use of isocyanate-based resins in the production of customized medical devices and implants.
Looking ahead, the evolution of isocyanates in medical devices is likely to focus on further enhancing biocompatibility, developing smart materials with responsive properties, and exploring sustainable production methods. The integration of nanotechnology and the development of hybrid materials combining isocyanates with other advanced polymers are expected to drive innovation in this field, potentially revolutionizing the design and functionality of future medical devices.
Market Demand Analysis
The market demand for isocyanate-based medical devices has been steadily growing, driven by the unique properties these materials offer in various healthcare applications. Isocyanates, particularly polyurethanes derived from them, are widely used in medical devices due to their versatility, durability, and biocompatibility. The global medical polyurethane market, which heavily relies on isocyanates, is projected to expand significantly in the coming years.
One of the primary drivers of market demand is the increasing prevalence of chronic diseases and the aging population worldwide. This demographic shift has led to a higher demand for long-term medical devices, many of which incorporate isocyanate-based materials. Cardiovascular devices, such as pacemakers and artificial heart valves, often utilize polyurethane components for their excellent mechanical properties and resistance to degradation in the body.
The wound care segment represents another substantial market for isocyanate-based products. Advanced wound dressings made from polyurethane foams offer superior absorption capabilities and create an optimal healing environment. As the incidence of chronic wounds rises, particularly in diabetic patients, the demand for these specialized dressings continues to grow.
In the orthopedic sector, isocyanate-derived materials are gaining traction in applications such as joint replacements and spinal implants. The ability to fine-tune the mechanical properties of polyurethanes makes them attractive for creating devices that can mimic natural tissue behavior, potentially improving patient outcomes and device longevity.
The trend towards minimally invasive procedures has also boosted the demand for isocyanate-based medical devices. Catheters, guidewires, and other interventional tools often incorporate polyurethane components due to their flexibility, strength, and ability to be manufactured in complex shapes.
However, the market is not without challenges. Concerns over the potential health risks associated with certain isocyanates have led to increased scrutiny and regulatory oversight. This has spurred innovation in the development of safer, low-emission isocyanates and alternative chemistries that maintain the desirable properties of traditional isocyanate-based materials.
Sustainability considerations are also shaping market demand. There is a growing interest in bio-based isocyanates and polyurethanes derived from renewable resources, aligning with broader healthcare industry trends towards environmentally friendly materials and practices.
As medical technology advances, the demand for customized and patient-specific devices is rising. Isocyanate-based materials, with their adaptable properties and compatibility with 3D printing technologies, are well-positioned to meet this emerging need, potentially opening new market opportunities in personalized medicine.
One of the primary drivers of market demand is the increasing prevalence of chronic diseases and the aging population worldwide. This demographic shift has led to a higher demand for long-term medical devices, many of which incorporate isocyanate-based materials. Cardiovascular devices, such as pacemakers and artificial heart valves, often utilize polyurethane components for their excellent mechanical properties and resistance to degradation in the body.
The wound care segment represents another substantial market for isocyanate-based products. Advanced wound dressings made from polyurethane foams offer superior absorption capabilities and create an optimal healing environment. As the incidence of chronic wounds rises, particularly in diabetic patients, the demand for these specialized dressings continues to grow.
In the orthopedic sector, isocyanate-derived materials are gaining traction in applications such as joint replacements and spinal implants. The ability to fine-tune the mechanical properties of polyurethanes makes them attractive for creating devices that can mimic natural tissue behavior, potentially improving patient outcomes and device longevity.
The trend towards minimally invasive procedures has also boosted the demand for isocyanate-based medical devices. Catheters, guidewires, and other interventional tools often incorporate polyurethane components due to their flexibility, strength, and ability to be manufactured in complex shapes.
However, the market is not without challenges. Concerns over the potential health risks associated with certain isocyanates have led to increased scrutiny and regulatory oversight. This has spurred innovation in the development of safer, low-emission isocyanates and alternative chemistries that maintain the desirable properties of traditional isocyanate-based materials.
Sustainability considerations are also shaping market demand. There is a growing interest in bio-based isocyanates and polyurethanes derived from renewable resources, aligning with broader healthcare industry trends towards environmentally friendly materials and practices.
As medical technology advances, the demand for customized and patient-specific devices is rising. Isocyanate-based materials, with their adaptable properties and compatibility with 3D printing technologies, are well-positioned to meet this emerging need, potentially opening new market opportunities in personalized medicine.
Current Challenges in Isocyanate Usage
Despite the widespread use of isocyanates in medical devices, several challenges persist in their application. One of the primary concerns is the potential toxicity and health risks associated with isocyanate exposure. Isocyanates are known respiratory sensitizers and can cause occupational asthma, skin irritation, and other allergic reactions. This poses significant risks not only to workers involved in the manufacturing process but also to healthcare professionals and patients who come into contact with isocyanate-containing medical devices.
Another challenge lies in the environmental impact of isocyanate production and disposal. The manufacturing process of isocyanates often involves the use of hazardous chemicals and generates potentially harmful byproducts. Additionally, the disposal of medical devices containing isocyanates can contribute to environmental pollution if not managed properly, raising concerns about long-term ecological effects.
The regulatory landscape surrounding isocyanate use in medical devices is becoming increasingly complex. Stringent regulations and guidelines imposed by various health and safety organizations worldwide require manufacturers to adhere to strict safety standards and testing protocols. Compliance with these regulations can be time-consuming and costly, potentially hindering innovation and market entry for smaller companies.
Furthermore, there is a growing demand for alternatives to isocyanate-based materials in medical devices. This shift is driven by both health concerns and the push for more sustainable and environmentally friendly options. However, finding suitable replacements that offer the same level of performance, durability, and cost-effectiveness as isocyanates remains a significant challenge for the industry.
The variability in isocyanate reactivity and stability presents another hurdle in their use for medical applications. Isocyanates are highly reactive compounds, and their properties can be affected by factors such as temperature, humidity, and storage conditions. This variability can lead to inconsistencies in product performance and shelf life, necessitating careful control and monitoring throughout the manufacturing and storage processes.
Lastly, the biocompatibility of isocyanate-based materials in long-term implantable devices remains a concern. While many isocyanate-derived polymers have shown good short-term biocompatibility, their long-term effects on the human body, particularly in implantable devices, are not fully understood. This uncertainty necessitates extensive research and clinical trials, which can be both time-consuming and expensive for medical device manufacturers.
Another challenge lies in the environmental impact of isocyanate production and disposal. The manufacturing process of isocyanates often involves the use of hazardous chemicals and generates potentially harmful byproducts. Additionally, the disposal of medical devices containing isocyanates can contribute to environmental pollution if not managed properly, raising concerns about long-term ecological effects.
The regulatory landscape surrounding isocyanate use in medical devices is becoming increasingly complex. Stringent regulations and guidelines imposed by various health and safety organizations worldwide require manufacturers to adhere to strict safety standards and testing protocols. Compliance with these regulations can be time-consuming and costly, potentially hindering innovation and market entry for smaller companies.
Furthermore, there is a growing demand for alternatives to isocyanate-based materials in medical devices. This shift is driven by both health concerns and the push for more sustainable and environmentally friendly options. However, finding suitable replacements that offer the same level of performance, durability, and cost-effectiveness as isocyanates remains a significant challenge for the industry.
The variability in isocyanate reactivity and stability presents another hurdle in their use for medical applications. Isocyanates are highly reactive compounds, and their properties can be affected by factors such as temperature, humidity, and storage conditions. This variability can lead to inconsistencies in product performance and shelf life, necessitating careful control and monitoring throughout the manufacturing and storage processes.
Lastly, the biocompatibility of isocyanate-based materials in long-term implantable devices remains a concern. While many isocyanate-derived polymers have shown good short-term biocompatibility, their long-term effects on the human body, particularly in implantable devices, are not fully understood. This uncertainty necessitates extensive research and clinical trials, which can be both time-consuming and expensive for medical device manufacturers.
Existing Isocyanate Solutions
01 Synthesis and production of isocyanates
Various methods and processes for synthesizing and producing isocyanates are described. These include novel catalysts, reaction conditions, and precursor materials to improve yield, purity, and efficiency in isocyanate production.- Synthesis and production of isocyanates: Various methods and processes for synthesizing and producing isocyanates are described. These include novel reaction pathways, catalysts, and production techniques to improve yield, efficiency, and purity of isocyanate compounds.
- Applications of isocyanates in polymer chemistry: Isocyanates play a crucial role in polymer chemistry, particularly in the production of polyurethanes. They are used in various applications such as coatings, adhesives, foams, and elastomers. The patents describe specific formulations and processes for utilizing isocyanates in these applications.
- Modification and functionalization of isocyanates: Techniques for modifying and functionalizing isocyanates to enhance their properties or create new compounds are presented. This includes the addition of specific functional groups, creation of isocyanate derivatives, and development of blocked isocyanates for controlled reactivity.
- Isocyanate-based catalysts and reaction systems: Isocyanates are utilized in the development of novel catalysts and reaction systems. These innovations aim to improve reaction rates, selectivity, and overall efficiency in various chemical processes, including polymerization and organic synthesis.
- Safety and handling of isocyanates: Given the reactive nature of isocyanates, patents address safety concerns and handling procedures. This includes methods for reducing toxicity, improving storage stability, and developing safer formulations for industrial use. Techniques for detecting and measuring isocyanate exposure are also described.
02 Applications of isocyanates in polymer chemistry
Isocyanates are widely used in polymer chemistry, particularly in the production of polyurethanes. The patents describe various applications, including coatings, adhesives, foams, and elastomers, as well as novel formulations and processing techniques.Expand Specific Solutions03 Isocyanate-based catalysts and additives
Several patents focus on the development of isocyanate-based catalysts and additives for various chemical processes. These include novel catalyst systems, stabilizers, and modifiers that enhance reaction rates, selectivity, or product properties.Expand Specific Solutions04 Safety and handling of isocyanates
Given the reactive nature of isocyanates, patents in this category address safety concerns and handling procedures. This includes methods for reducing toxicity, improving storage stability, and developing safer formulations for industrial use.Expand Specific Solutions05 Isocyanate-free alternatives and substitutes
Some patents focus on developing alternatives or substitutes for isocyanates in various applications. This includes novel chemistries, bio-based materials, and modified processes that aim to reduce or eliminate the use of traditional isocyanates while maintaining desired product properties.Expand Specific Solutions
Key Players in Medical Isocyanates
The isocyanate use in medical devices market is in a growth phase, driven by increasing demand for advanced medical technologies. The market size is expanding, with projections indicating significant growth potential in the coming years. Technologically, the field is advancing rapidly, with major players like Wanhua Chemical, BASF, Covestro, and Bayer leading innovations. These companies are developing novel isocyanate-based materials with enhanced biocompatibility and performance for various medical applications. Academic institutions such as Sichuan University and Nanyang Technological University are also contributing to research and development efforts, pushing the boundaries of isocyanate technology in medical devices. The competitive landscape is characterized by a mix of established chemical companies and specialized medical device manufacturers collaborating to bring new products to market.
BASF Corp.
Technical Solution: BASF has developed innovative isocyanate-based materials for medical devices, focusing on polyurethane formulations with enhanced biocompatibility and durability. Their recent innovations include a novel aliphatic isocyanate-terminated prepolymer system that offers improved hydrolytic stability and reduced potential for harmful degradation products[1]. This technology enables the production of medical-grade polyurethanes with superior mechanical properties and long-term performance in implantable devices. BASF has also introduced a range of water-based polyurethane dispersions specifically designed for medical applications, providing a more environmentally friendly and safer alternative to solvent-based systems[3].
Strengths: Extensive R&D capabilities, wide range of isocyanate chemistries, strong focus on sustainability. Weaknesses: Regulatory challenges in bringing new materials to market, potential competition from specialized medical material manufacturers.
Covestro Deutschland AG
Technical Solution: Covestro has made significant strides in developing isocyanate-based materials for medical devices, with a focus on thermoplastic polyurethanes (TPUs). Their latest innovation is a series of polycarbonate-based TPUs that offer exceptional biocompatibility and resistance to oxidation and hydrolysis[2]. These materials are particularly suitable for long-term implantable devices. Covestro has also developed a proprietary technology for producing ultra-high molecular weight TPUs, which exhibit improved mechanical strength and wear resistance, crucial for orthopedic and cardiovascular applications[4]. Additionally, they have introduced a range of aliphatic isocyanate-based coatings that provide excellent chemical resistance and durability for medical equipment and devices[5].
Strengths: Strong expertise in polyurethane chemistry, broad product portfolio, focus on sustainable solutions. Weaknesses: Potential limitations in specialized medical-grade formulations, competition from established medical device material suppliers.
Innovative Isocyanate Applications
Medical adhesive
PatentActiveUS8273847B2
Innovation
- A medical adhesive comprising a hydrophilic urethane prepolymer formed by reacting a fluorine-containing nonaromatic polyisocyanate with a hydrophilic polyol and a phenolic radical scavenger, which enhances reactivity and stability while preventing degradation.
Elongated medical device, and method for producing elongated medical device
PatentWO2024004573A1
Innovation
- A medical device with a base layer composed of an isocyanate compound and a top layer containing a copolymer with hydrophilic and cyclic carbonate structures, which forms covalent bonds with the base layer, enhancing adhesion and lubricity.
Regulatory Framework
The regulatory framework surrounding the use of isocyanates in medical devices has evolved significantly in recent years, reflecting growing concerns about patient safety and environmental impact. In the United States, the Food and Drug Administration (FDA) has implemented stringent guidelines for the use of isocyanates in medical devices, particularly focusing on their potential to cause allergic reactions and respiratory issues.
The FDA requires manufacturers to conduct thorough biocompatibility testing and risk assessments for any medical device containing isocyanates. This includes evaluating the potential for leaching of isocyanate compounds and their degradation products over the device's lifetime. Additionally, manufacturers must provide detailed information on the chemical composition, manufacturing processes, and sterilization methods used for isocyanate-containing devices.
In the European Union, the Medical Device Regulation (MDR) has introduced more rigorous requirements for the use of potentially hazardous substances in medical devices. Isocyanates fall under the category of substances of concern, necessitating additional documentation and justification for their use. Manufacturers must demonstrate that the benefits of using isocyanates outweigh any potential risks and that no suitable alternatives are available.
The International Organization for Standardization (ISO) has also developed specific standards related to the use of isocyanates in medical devices. ISO 10993-1, which addresses the biological evaluation of medical devices, includes guidelines for assessing the safety of materials containing isocyanates. This standard emphasizes the importance of considering the entire lifecycle of the device, from manufacturing to disposal.
Regulatory bodies worldwide are increasingly focusing on the environmental impact of medical devices. This has led to stricter regulations on the disposal and recycling of isocyanate-containing devices. Manufacturers are now required to provide detailed end-of-life plans and consider the potential environmental consequences of their products.
As innovations in isocyanate use continue to emerge, regulatory frameworks are adapting to keep pace. There is a growing trend towards harmonization of regulations across different regions, aiming to streamline the approval process for new medical devices while maintaining high safety standards. This includes efforts to establish common testing protocols and safety thresholds for isocyanates in medical applications.
The regulatory landscape also reflects an increased emphasis on transparency and traceability. Manufacturers are required to maintain comprehensive documentation of their isocyanate sourcing, processing, and quality control measures. This information must be readily available for regulatory inspections and audits, ensuring accountability throughout the supply chain.
The FDA requires manufacturers to conduct thorough biocompatibility testing and risk assessments for any medical device containing isocyanates. This includes evaluating the potential for leaching of isocyanate compounds and their degradation products over the device's lifetime. Additionally, manufacturers must provide detailed information on the chemical composition, manufacturing processes, and sterilization methods used for isocyanate-containing devices.
In the European Union, the Medical Device Regulation (MDR) has introduced more rigorous requirements for the use of potentially hazardous substances in medical devices. Isocyanates fall under the category of substances of concern, necessitating additional documentation and justification for their use. Manufacturers must demonstrate that the benefits of using isocyanates outweigh any potential risks and that no suitable alternatives are available.
The International Organization for Standardization (ISO) has also developed specific standards related to the use of isocyanates in medical devices. ISO 10993-1, which addresses the biological evaluation of medical devices, includes guidelines for assessing the safety of materials containing isocyanates. This standard emphasizes the importance of considering the entire lifecycle of the device, from manufacturing to disposal.
Regulatory bodies worldwide are increasingly focusing on the environmental impact of medical devices. This has led to stricter regulations on the disposal and recycling of isocyanate-containing devices. Manufacturers are now required to provide detailed end-of-life plans and consider the potential environmental consequences of their products.
As innovations in isocyanate use continue to emerge, regulatory frameworks are adapting to keep pace. There is a growing trend towards harmonization of regulations across different regions, aiming to streamline the approval process for new medical devices while maintaining high safety standards. This includes efforts to establish common testing protocols and safety thresholds for isocyanates in medical applications.
The regulatory landscape also reflects an increased emphasis on transparency and traceability. Manufacturers are required to maintain comprehensive documentation of their isocyanate sourcing, processing, and quality control measures. This information must be readily available for regulatory inspections and audits, ensuring accountability throughout the supply chain.
Biocompatibility Assessment
Biocompatibility assessment is a critical aspect of evaluating isocyanate use in medical devices, particularly in light of recent innovations. This assessment involves a comprehensive evaluation of the material's interaction with biological systems to ensure safety and efficacy in medical applications.
The primary focus of biocompatibility assessment for isocyanate-based materials is to determine their potential for adverse reactions when in contact with human tissues, blood, or other bodily fluids. Recent advancements have led to more sophisticated testing methods that can provide a more accurate prediction of in vivo performance.
One significant innovation in biocompatibility assessment is the development of in vitro cell culture models that closely mimic human tissue responses. These models allow for the evaluation of cytotoxicity, inflammatory responses, and potential genotoxicity of isocyanate-derived materials without the need for extensive animal testing. This approach not only accelerates the assessment process but also aligns with ethical considerations in medical research.
Advanced surface characterization techniques have also played a crucial role in enhancing biocompatibility assessments. High-resolution imaging methods, such as atomic force microscopy and X-ray photoelectron spectroscopy, enable researchers to analyze the surface properties of isocyanate-based materials at the nanoscale. This level of detail provides valuable insights into how these surfaces interact with proteins and cells, which is essential for predicting biocompatibility.
Recent innovations have also focused on developing more sensitive analytical methods for detecting trace amounts of unreacted isocyanates or their degradation products. These methods are crucial for ensuring that medical devices do not release harmful substances over time. Techniques such as liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS) have been optimized for this purpose, allowing for the detection of extremely low concentrations of potential contaminants.
The integration of computational modeling into biocompatibility assessment has been another significant advancement. Machine learning algorithms and molecular dynamics simulations are now being employed to predict the behavior of isocyanate-based materials in biological environments. These computational approaches can help identify potential issues early in the development process, reducing the time and resources required for physical testing.
Furthermore, long-term biocompatibility studies have been enhanced through the use of implantable sensors and real-time monitoring systems. These technologies allow researchers to gather continuous data on the performance of isocyanate-containing medical devices in vivo, providing a more comprehensive understanding of their long-term safety and efficacy.
In conclusion, recent innovations in biocompatibility assessment for isocyanate use in medical devices have significantly improved the accuracy, efficiency, and depth of evaluation. These advancements contribute to the development of safer and more effective medical devices, ultimately benefiting patient care and outcomes.
The primary focus of biocompatibility assessment for isocyanate-based materials is to determine their potential for adverse reactions when in contact with human tissues, blood, or other bodily fluids. Recent advancements have led to more sophisticated testing methods that can provide a more accurate prediction of in vivo performance.
One significant innovation in biocompatibility assessment is the development of in vitro cell culture models that closely mimic human tissue responses. These models allow for the evaluation of cytotoxicity, inflammatory responses, and potential genotoxicity of isocyanate-derived materials without the need for extensive animal testing. This approach not only accelerates the assessment process but also aligns with ethical considerations in medical research.
Advanced surface characterization techniques have also played a crucial role in enhancing biocompatibility assessments. High-resolution imaging methods, such as atomic force microscopy and X-ray photoelectron spectroscopy, enable researchers to analyze the surface properties of isocyanate-based materials at the nanoscale. This level of detail provides valuable insights into how these surfaces interact with proteins and cells, which is essential for predicting biocompatibility.
Recent innovations have also focused on developing more sensitive analytical methods for detecting trace amounts of unreacted isocyanates or their degradation products. These methods are crucial for ensuring that medical devices do not release harmful substances over time. Techniques such as liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS) have been optimized for this purpose, allowing for the detection of extremely low concentrations of potential contaminants.
The integration of computational modeling into biocompatibility assessment has been another significant advancement. Machine learning algorithms and molecular dynamics simulations are now being employed to predict the behavior of isocyanate-based materials in biological environments. These computational approaches can help identify potential issues early in the development process, reducing the time and resources required for physical testing.
Furthermore, long-term biocompatibility studies have been enhanced through the use of implantable sensors and real-time monitoring systems. These technologies allow researchers to gather continuous data on the performance of isocyanate-containing medical devices in vivo, providing a more comprehensive understanding of their long-term safety and efficacy.
In conclusion, recent innovations in biocompatibility assessment for isocyanate use in medical devices have significantly improved the accuracy, efficiency, and depth of evaluation. These advancements contribute to the development of safer and more effective medical devices, ultimately benefiting patient care and outcomes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!