Analyzing Hypochlorous Acid's Corrosion Potential on Surfaces
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HOCl Corrosion Background
Hypochlorous acid (HOCl) has been a subject of interest in various industries due to its potent disinfectant properties. However, its corrosive nature has raised concerns about its long-term effects on surfaces. The study of HOCl's corrosion potential is crucial for understanding its impact on materials and developing appropriate mitigation strategies.
HOCl is a weak acid formed when chlorine dissolves in water. It has been used for over a century in water treatment, food processing, and healthcare settings. The compound's ability to effectively kill bacteria, viruses, and fungi has made it a popular choice for disinfection. However, its corrosive properties have been a persistent challenge, particularly in industrial applications where equipment longevity is paramount.
The corrosion potential of HOCl is primarily attributed to its oxidizing nature. As a strong oxidizer, it can react with many materials, particularly metals, leading to degradation over time. The pH of the HOCl solution plays a significant role in its corrosivity, with more acidic solutions generally being more corrosive. Temperature and concentration are also key factors influencing the rate and extent of corrosion.
Historical studies have shown that HOCl can cause pitting corrosion in stainless steel, a material often chosen for its corrosion resistance. This type of localized corrosion can be particularly problematic as it can lead to equipment failure without visible surface deterioration. Other materials, such as copper and its alloys, have also shown susceptibility to HOCl-induced corrosion.
The impact of HOCl corrosion extends beyond metal surfaces. Polymers and elastomers used in seals, gaskets, and linings can also be affected, potentially leading to leaks or contamination in critical systems. This has implications for industries ranging from water treatment plants to food processing facilities, where maintaining the integrity of equipment is essential for operational safety and product quality.
Recent advancements in materials science and surface engineering have led to the development of more resistant materials and protective coatings. These innovations aim to mitigate the corrosive effects of HOCl while maintaining its disinfectant properties. However, the balance between effectiveness and material compatibility remains a challenge, driving ongoing research in this field.
Understanding the mechanisms of HOCl-induced corrosion is crucial for developing effective prevention strategies. This includes not only the selection of appropriate materials but also the implementation of proper maintenance protocols and monitoring systems. As industries continue to rely on HOCl for its disinfectant properties, the need for comprehensive corrosion management strategies becomes increasingly important.
HOCl is a weak acid formed when chlorine dissolves in water. It has been used for over a century in water treatment, food processing, and healthcare settings. The compound's ability to effectively kill bacteria, viruses, and fungi has made it a popular choice for disinfection. However, its corrosive properties have been a persistent challenge, particularly in industrial applications where equipment longevity is paramount.
The corrosion potential of HOCl is primarily attributed to its oxidizing nature. As a strong oxidizer, it can react with many materials, particularly metals, leading to degradation over time. The pH of the HOCl solution plays a significant role in its corrosivity, with more acidic solutions generally being more corrosive. Temperature and concentration are also key factors influencing the rate and extent of corrosion.
Historical studies have shown that HOCl can cause pitting corrosion in stainless steel, a material often chosen for its corrosion resistance. This type of localized corrosion can be particularly problematic as it can lead to equipment failure without visible surface deterioration. Other materials, such as copper and its alloys, have also shown susceptibility to HOCl-induced corrosion.
The impact of HOCl corrosion extends beyond metal surfaces. Polymers and elastomers used in seals, gaskets, and linings can also be affected, potentially leading to leaks or contamination in critical systems. This has implications for industries ranging from water treatment plants to food processing facilities, where maintaining the integrity of equipment is essential for operational safety and product quality.
Recent advancements in materials science and surface engineering have led to the development of more resistant materials and protective coatings. These innovations aim to mitigate the corrosive effects of HOCl while maintaining its disinfectant properties. However, the balance between effectiveness and material compatibility remains a challenge, driving ongoing research in this field.
Understanding the mechanisms of HOCl-induced corrosion is crucial for developing effective prevention strategies. This includes not only the selection of appropriate materials but also the implementation of proper maintenance protocols and monitoring systems. As industries continue to rely on HOCl for its disinfectant properties, the need for comprehensive corrosion management strategies becomes increasingly important.
Market Demand Analysis
The market demand for analyzing hypochlorous acid's corrosion potential on surfaces has been steadily growing across various industries. This demand is primarily driven by the increasing use of hypochlorous acid as a disinfectant and sanitizer, particularly in healthcare, food processing, and water treatment sectors.
In the healthcare industry, there is a significant need for understanding the corrosive effects of hypochlorous acid on medical equipment and surfaces. Hospitals, clinics, and other healthcare facilities are increasingly adopting hypochlorous acid-based disinfection methods due to their effectiveness against a wide range of pathogens. However, concerns about potential damage to sensitive medical devices and infrastructure have created a strong market for corrosion analysis and mitigation solutions.
The food processing industry also contributes substantially to the market demand. As food safety regulations become more stringent, manufacturers are turning to hypochlorous acid for sanitizing food contact surfaces and equipment. This shift has led to a growing need for comprehensive studies on the long-term effects of hypochlorous acid exposure on various materials used in food processing facilities.
Water treatment plants represent another significant market segment. The use of hypochlorous acid in water disinfection processes has raised questions about its impact on distribution systems and storage tanks. Municipalities and water management companies are investing in research to assess and mitigate potential corrosion risks associated with hypochlorous acid treatment.
The COVID-19 pandemic has further accelerated the market demand for hypochlorous acid corrosion analysis. The widespread adoption of enhanced cleaning and disinfection protocols in public spaces, transportation systems, and commercial buildings has increased the use of hypochlorous acid-based products. This surge in usage has heightened awareness of potential long-term consequences on infrastructure and equipment, driving demand for corrosion studies and protective solutions.
Environmental concerns and sustainability initiatives are also shaping market trends. As industries seek more eco-friendly disinfection methods, hypochlorous acid has gained attention due to its lower environmental impact compared to traditional chlorine-based disinfectants. However, this shift has created a parallel demand for understanding the long-term environmental effects of hypochlorous acid, including its potential impact on soil and water ecosystems when released into the environment.
The market for analyzing hypochlorous acid's corrosion potential is expected to continue growing as more industries adopt this disinfection method. This growth is likely to be accompanied by increased investment in research and development of corrosion-resistant materials and protective coatings specifically designed to withstand hypochlorous acid exposure.
In the healthcare industry, there is a significant need for understanding the corrosive effects of hypochlorous acid on medical equipment and surfaces. Hospitals, clinics, and other healthcare facilities are increasingly adopting hypochlorous acid-based disinfection methods due to their effectiveness against a wide range of pathogens. However, concerns about potential damage to sensitive medical devices and infrastructure have created a strong market for corrosion analysis and mitigation solutions.
The food processing industry also contributes substantially to the market demand. As food safety regulations become more stringent, manufacturers are turning to hypochlorous acid for sanitizing food contact surfaces and equipment. This shift has led to a growing need for comprehensive studies on the long-term effects of hypochlorous acid exposure on various materials used in food processing facilities.
Water treatment plants represent another significant market segment. The use of hypochlorous acid in water disinfection processes has raised questions about its impact on distribution systems and storage tanks. Municipalities and water management companies are investing in research to assess and mitigate potential corrosion risks associated with hypochlorous acid treatment.
The COVID-19 pandemic has further accelerated the market demand for hypochlorous acid corrosion analysis. The widespread adoption of enhanced cleaning and disinfection protocols in public spaces, transportation systems, and commercial buildings has increased the use of hypochlorous acid-based products. This surge in usage has heightened awareness of potential long-term consequences on infrastructure and equipment, driving demand for corrosion studies and protective solutions.
Environmental concerns and sustainability initiatives are also shaping market trends. As industries seek more eco-friendly disinfection methods, hypochlorous acid has gained attention due to its lower environmental impact compared to traditional chlorine-based disinfectants. However, this shift has created a parallel demand for understanding the long-term environmental effects of hypochlorous acid, including its potential impact on soil and water ecosystems when released into the environment.
The market for analyzing hypochlorous acid's corrosion potential is expected to continue growing as more industries adopt this disinfection method. This growth is likely to be accompanied by increased investment in research and development of corrosion-resistant materials and protective coatings specifically designed to withstand hypochlorous acid exposure.
Current Challenges
The analysis of hypochlorous acid's corrosion potential on surfaces presents several significant challenges in current research and practical applications. One of the primary obstacles is the variability of surface materials and their diverse reactions to hypochlorous acid exposure. Different materials, such as metals, plastics, and composites, exhibit distinct corrosion mechanisms and rates when in contact with this powerful oxidizing agent. This heterogeneity complicates the development of universal corrosion prevention strategies and necessitates material-specific approaches.
Another critical challenge lies in accurately measuring and predicting the long-term effects of hypochlorous acid on surfaces. While short-term corrosion can often be observed and quantified, the cumulative impact of prolonged or intermittent exposure remains difficult to assess. This is particularly problematic in industries where equipment and infrastructure are expected to withstand years of service in hypochlorous acid-rich environments. The lack of standardized long-term testing protocols further exacerbates this issue, making it challenging to compare results across different studies and applications.
The concentration and pH of hypochlorous acid solutions pose additional complexities in corrosion analysis. The corrosive potential of hypochlorous acid can vary significantly depending on these factors, and maintaining consistent conditions across experiments and real-world applications is often challenging. Environmental factors such as temperature, humidity, and the presence of other chemical species can also influence corrosion rates, adding layers of complexity to the analysis.
Furthermore, the dynamic nature of surface-hypochlorous acid interactions presents a formidable challenge. As corrosion progresses, the surface characteristics of materials can change, potentially altering their susceptibility to further corrosion. This evolving interface makes it difficult to develop accurate predictive models and necessitates continuous monitoring and adaptive protection strategies.
The microscopic scale at which initial corrosion processes occur adds another dimension of difficulty to the analysis. Detecting and characterizing the early stages of corrosion often require advanced imaging and analytical techniques, which may not be readily available or easily implemented in all research or industrial settings. This limitation can lead to delayed detection of corrosion issues, potentially resulting in more severe damage and increased repair costs.
Lastly, the development of effective corrosion inhibitors specific to hypochlorous acid presents ongoing challenges. While various inhibitors exist for other corrosive agents, finding compounds that can effectively mitigate hypochlorous acid-induced corrosion without compromising its beneficial properties (such as disinfection efficacy) remains an active area of research. The need for environmentally friendly and cost-effective inhibitors further complicates this challenge, especially in applications where large-scale use is necessary.
Another critical challenge lies in accurately measuring and predicting the long-term effects of hypochlorous acid on surfaces. While short-term corrosion can often be observed and quantified, the cumulative impact of prolonged or intermittent exposure remains difficult to assess. This is particularly problematic in industries where equipment and infrastructure are expected to withstand years of service in hypochlorous acid-rich environments. The lack of standardized long-term testing protocols further exacerbates this issue, making it challenging to compare results across different studies and applications.
The concentration and pH of hypochlorous acid solutions pose additional complexities in corrosion analysis. The corrosive potential of hypochlorous acid can vary significantly depending on these factors, and maintaining consistent conditions across experiments and real-world applications is often challenging. Environmental factors such as temperature, humidity, and the presence of other chemical species can also influence corrosion rates, adding layers of complexity to the analysis.
Furthermore, the dynamic nature of surface-hypochlorous acid interactions presents a formidable challenge. As corrosion progresses, the surface characteristics of materials can change, potentially altering their susceptibility to further corrosion. This evolving interface makes it difficult to develop accurate predictive models and necessitates continuous monitoring and adaptive protection strategies.
The microscopic scale at which initial corrosion processes occur adds another dimension of difficulty to the analysis. Detecting and characterizing the early stages of corrosion often require advanced imaging and analytical techniques, which may not be readily available or easily implemented in all research or industrial settings. This limitation can lead to delayed detection of corrosion issues, potentially resulting in more severe damage and increased repair costs.
Lastly, the development of effective corrosion inhibitors specific to hypochlorous acid presents ongoing challenges. While various inhibitors exist for other corrosive agents, finding compounds that can effectively mitigate hypochlorous acid-induced corrosion without compromising its beneficial properties (such as disinfection efficacy) remains an active area of research. The need for environmentally friendly and cost-effective inhibitors further complicates this challenge, especially in applications where large-scale use is necessary.
Existing Mitigation Methods
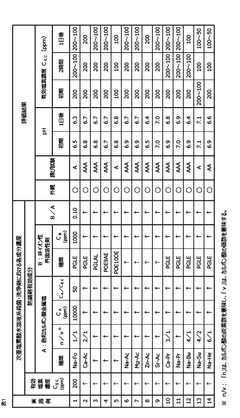
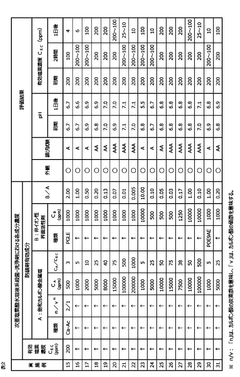
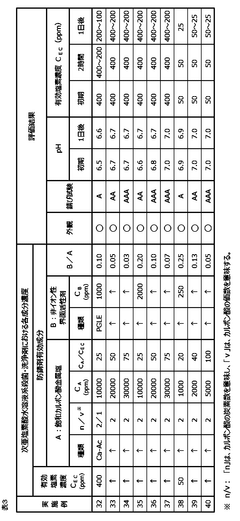
01 Corrosion inhibition methods for hypochlorous acid
Various methods are employed to inhibit the corrosive effects of hypochlorous acid on materials. These may include the use of protective coatings, corrosion-resistant alloys, or the addition of chemical inhibitors to reduce the acid's corrosive potential in industrial applications.- Corrosion inhibition methods for hypochlorous acid: Various methods are employed to inhibit the corrosive effects of hypochlorous acid on materials. These may include the use of protective coatings, corrosion-resistant alloys, or chemical additives that neutralize or mitigate the corrosive properties of the acid. Such techniques are crucial in industries where hypochlorous acid is commonly used or encountered.
- Material selection for hypochlorous acid resistance: Selecting appropriate materials that exhibit resistance to hypochlorous acid is essential in preventing corrosion. This may involve using specific metals, alloys, or polymers that demonstrate high resistance to oxidation and chemical attack by hypochlorous acid. The choice of materials can significantly impact the longevity and performance of equipment exposed to this corrosive substance.
- Monitoring and control systems for hypochlorous acid corrosion: Implementing monitoring and control systems helps in managing the corrosion potential of hypochlorous acid. These systems may include sensors for measuring acid concentration, pH levels, and corrosion rates, as well as automated dosing systems to maintain optimal conditions. Regular monitoring allows for timely interventions to prevent or mitigate corrosion issues.
- Surface treatment techniques for corrosion protection: Various surface treatment techniques can be applied to enhance the corrosion resistance of materials exposed to hypochlorous acid. These may include passivation, anodizing, or the application of specialized coatings. Such treatments create a protective layer on the material surface, reducing its susceptibility to corrosion by hypochlorous acid.
- Corrosion assessment and prediction methods: Developing and utilizing corrosion assessment and prediction methods is crucial for understanding and managing the corrosion potential of hypochlorous acid. These may include electrochemical testing, computational modeling, and long-term exposure studies. Such methods help in estimating corrosion rates, identifying vulnerable areas, and developing effective prevention strategies.
02 Material selection for hypochlorous acid resistance
Specific materials are chosen for their resistance to hypochlorous acid corrosion. This includes selecting appropriate metals, polymers, or composites that can withstand the oxidizing properties of the acid in various concentrations and environmental conditions.Expand Specific Solutions03 Monitoring and control of hypochlorous acid concentration
Systems and methods for monitoring and controlling the concentration of hypochlorous acid in solutions are developed to manage corrosion potential. This involves the use of sensors, automated dosing systems, and process control algorithms to maintain optimal acid levels.Expand Specific Solutions04 Surface treatment techniques for corrosion prevention
Various surface treatment techniques are applied to materials to enhance their resistance to hypochlorous acid corrosion. These may include passivation, anodizing, or the application of specialized coatings that create a barrier against the acid's corrosive effects.Expand Specific Solutions05 Neutralization and pH control strategies
Methods for neutralizing hypochlorous acid or controlling the pH of solutions containing it are developed to mitigate corrosion risks. This involves the use of buffering agents, pH adjusters, or chemical treatments to maintain a less corrosive environment.Expand Specific Solutions
Key Industry Players
The analysis of hypochlorous acid's corrosion potential on surfaces is in a mature stage of development, with a significant market size and established technological solutions. Key players in this field include Ecolab USA, Inc., Halliburton Energy Services, Inc., and The Clorox Co., who have developed advanced products and techniques to address corrosion issues. The market is characterized by ongoing research and innovation, particularly in industries such as water treatment, oil and gas, and healthcare. Companies like Henkel AG & Co. KGaA and NIPPON STEEL CORP. are also contributing to the advancement of corrosion-resistant materials and coatings. The technology's maturity is evident in the diverse applications across multiple sectors, indicating a well-established understanding of hypochlorous acid's properties and its interactions with various surfaces.
Ecolab USA, Inc.
Technical Solution: Ecolab has developed advanced corrosion inhibition technologies for hypochlorous acid applications. Their approach involves using proprietary blends of organic and inorganic inhibitors that form protective films on metal surfaces[1]. These formulations are designed to maintain the antimicrobial efficacy of hypochlorous acid while significantly reducing its corrosive effects on various materials, including stainless steel, copper, and aluminum[2]. Ecolab's solutions also incorporate pH stabilizers to optimize the balance between disinfection power and material compatibility[3].
Strengths: Comprehensive corrosion protection across multiple materials; maintains antimicrobial efficacy. Weaknesses: May require frequent reapplication; potential for increased costs due to proprietary formulations.
Henkel AG & Co. KGaA
Technical Solution: Henkel has pioneered a novel approach to mitigating hypochlorous acid corrosion through the development of advanced surface coatings. Their technology utilizes a multi-layer polymer system that creates a barrier resistant to both chemical attack and mechanical wear[4]. The coating is designed to be thin enough to maintain the original surface properties while providing long-lasting protection against hypochlorous acid. Henkel's research has shown that these coatings can extend the lifespan of metal components exposed to hypochlorous acid by up to 300%[5]. Additionally, they have developed specialized application methods to ensure uniform coverage on complex geometries[6].
Strengths: Long-lasting protection; minimal impact on surface properties; applicable to complex geometries. Weaknesses: Initial application cost may be high; potential for coating damage in high-wear areas.
Advanced Corrosion Research
Rust preventative for hypochlorous acid aqueous solution, fungicide and method for preparing same
PatentPendingEP4534724A1
Innovation
- A corrosion inhibitor comprising a metal salt of a carboxylic acid with 1 to 9 carbon atoms and a compound with a triazole skeleton, applied at a specific ratio, effectively suppresses corrosion on metal surfaces and maintains the effective chlorine concentration and neutral pH of hypochlorous acid aqueous solutions.
Corrosion inhibitor for hypochlorous acid aqueous solution, bactericidal detergent having corrosion inhibiting effect, and preparation method therefor
PatentWO2022158522A1
Innovation
- A rust preventive agent for hypochlorous acid aqueous solutions comprising a metal salt of formic acid or saturated carboxylic acid combined with a nonionic surfactant, which is added to the solution at specific ratios to prevent rust on metal surfaces while maintaining effective chlorine concentration and pH neutrality.
Material Compatibility
The compatibility of hypochlorous acid (HOCl) with various materials is a critical consideration when analyzing its corrosion potential on surfaces. HOCl, known for its strong oxidizing properties, can interact differently with various materials, leading to varying degrees of corrosion or degradation.
Metals are particularly susceptible to HOCl-induced corrosion. Stainless steel, widely used in industrial applications, exhibits varying levels of resistance depending on its grade and composition. Lower-grade stainless steels may experience pitting or crevice corrosion when exposed to HOCl, especially at higher concentrations or prolonged contact times. In contrast, high-grade stainless steels, such as 316L or 904L, demonstrate superior resistance to HOCl-induced corrosion due to their higher molybdenum content.
Aluminum and its alloys are generally more vulnerable to HOCl corrosion compared to stainless steel. The protective oxide layer on aluminum surfaces can be compromised by HOCl, leading to accelerated corrosion rates. This is particularly problematic in applications where aluminum components are regularly exposed to HOCl-based disinfectants or cleaning solutions.
Copper and copper alloys exhibit moderate resistance to HOCl corrosion. While they may not corrode as rapidly as some other metals, prolonged exposure can lead to surface discoloration and gradual material degradation. This is an important consideration in plumbing systems or heat exchangers where copper is commonly used.
Polymers and elastomers show varying degrees of compatibility with HOCl. Fluoropolymers, such as PTFE (Teflon) and PVDF, demonstrate excellent resistance to HOCl and are often used in applications requiring prolonged exposure. However, some elastomers, particularly those containing unsaturated bonds, may degrade when exposed to HOCl, leading to changes in mechanical properties or leaching of compounds.
Concrete and cementitious materials can also be affected by HOCl exposure. While the initial alkalinity of concrete provides some protection, prolonged contact with HOCl can lead to the degradation of the cement matrix, potentially compromising structural integrity. This is particularly relevant in water treatment facilities or swimming pools where HOCl is commonly used as a disinfectant.
Glass and ceramic materials generally exhibit good resistance to HOCl corrosion. Their inert nature and lack of reactive sites make them suitable for applications involving HOCl storage or handling. However, certain specialized glasses or ceramics with specific coatings or additives may show altered behavior when exposed to HOCl.
Understanding these material compatibility issues is crucial for designing systems and selecting appropriate materials for applications involving HOCl. Factors such as concentration, temperature, pH, and exposure duration must be carefully considered to mitigate corrosion risks and ensure the longevity and safety of equipment and structures in contact with HOCl.
Metals are particularly susceptible to HOCl-induced corrosion. Stainless steel, widely used in industrial applications, exhibits varying levels of resistance depending on its grade and composition. Lower-grade stainless steels may experience pitting or crevice corrosion when exposed to HOCl, especially at higher concentrations or prolonged contact times. In contrast, high-grade stainless steels, such as 316L or 904L, demonstrate superior resistance to HOCl-induced corrosion due to their higher molybdenum content.
Aluminum and its alloys are generally more vulnerable to HOCl corrosion compared to stainless steel. The protective oxide layer on aluminum surfaces can be compromised by HOCl, leading to accelerated corrosion rates. This is particularly problematic in applications where aluminum components are regularly exposed to HOCl-based disinfectants or cleaning solutions.
Copper and copper alloys exhibit moderate resistance to HOCl corrosion. While they may not corrode as rapidly as some other metals, prolonged exposure can lead to surface discoloration and gradual material degradation. This is an important consideration in plumbing systems or heat exchangers where copper is commonly used.
Polymers and elastomers show varying degrees of compatibility with HOCl. Fluoropolymers, such as PTFE (Teflon) and PVDF, demonstrate excellent resistance to HOCl and are often used in applications requiring prolonged exposure. However, some elastomers, particularly those containing unsaturated bonds, may degrade when exposed to HOCl, leading to changes in mechanical properties or leaching of compounds.
Concrete and cementitious materials can also be affected by HOCl exposure. While the initial alkalinity of concrete provides some protection, prolonged contact with HOCl can lead to the degradation of the cement matrix, potentially compromising structural integrity. This is particularly relevant in water treatment facilities or swimming pools where HOCl is commonly used as a disinfectant.
Glass and ceramic materials generally exhibit good resistance to HOCl corrosion. Their inert nature and lack of reactive sites make them suitable for applications involving HOCl storage or handling. However, certain specialized glasses or ceramics with specific coatings or additives may show altered behavior when exposed to HOCl.
Understanding these material compatibility issues is crucial for designing systems and selecting appropriate materials for applications involving HOCl. Factors such as concentration, temperature, pH, and exposure duration must be carefully considered to mitigate corrosion risks and ensure the longevity and safety of equipment and structures in contact with HOCl.
Environmental Impact
The environmental impact of hypochlorous acid (HOCl) is a critical consideration when analyzing its corrosion potential on surfaces. HOCl is widely used as a disinfectant and sanitizer in various industries, including water treatment, healthcare, and food processing. While it is highly effective in killing pathogens, its potential environmental consequences must be carefully evaluated.
HOCl naturally decomposes into harmless byproducts, primarily salt and water, which is a significant advantage from an environmental perspective. This characteristic makes it a more eco-friendly alternative to many traditional disinfectants that may leave harmful residues or produce toxic byproducts. The rapid decomposition of HOCl also means that it does not persist in the environment, reducing the risk of long-term ecological damage.
However, the production and use of HOCl can have indirect environmental impacts. The energy required for its generation, typically through electrolysis of salt water, contributes to carbon emissions if non-renewable energy sources are used. Additionally, the salt used in the production process may come from mining operations, which can have their own environmental footprint.
When considering the corrosion potential of HOCl on surfaces, it is essential to assess the environmental implications of material degradation. Corrosion can lead to the release of metal ions and other compounds into the surrounding environment, potentially affecting soil and water quality. This is particularly concerning in applications where HOCl is used in large quantities or in sensitive ecosystems.
The pH of HOCl solutions is another factor that influences its environmental impact. While HOCl is most effective as a disinfectant at a slightly acidic pH, prolonged exposure to acidic conditions can accelerate corrosion and potentially harm aquatic life if released into water bodies. Proper pH control and neutralization procedures are crucial to mitigate these risks.
It is worth noting that the environmental impact of HOCl's corrosion potential varies depending on the specific surfaces and materials involved. Some materials may be more resistant to HOCl-induced corrosion, while others may degrade more rapidly. This variability underscores the importance of conducting thorough material compatibility studies and implementing appropriate protective measures to minimize environmental risks.
In conclusion, while HOCl offers several environmental benefits as a disinfectant, its corrosion potential must be carefully managed to prevent unintended ecological consequences. Balancing the need for effective sanitation with environmental protection requires ongoing research, proper application techniques, and the development of corrosion-resistant materials suitable for use with HOCl.
HOCl naturally decomposes into harmless byproducts, primarily salt and water, which is a significant advantage from an environmental perspective. This characteristic makes it a more eco-friendly alternative to many traditional disinfectants that may leave harmful residues or produce toxic byproducts. The rapid decomposition of HOCl also means that it does not persist in the environment, reducing the risk of long-term ecological damage.
However, the production and use of HOCl can have indirect environmental impacts. The energy required for its generation, typically through electrolysis of salt water, contributes to carbon emissions if non-renewable energy sources are used. Additionally, the salt used in the production process may come from mining operations, which can have their own environmental footprint.
When considering the corrosion potential of HOCl on surfaces, it is essential to assess the environmental implications of material degradation. Corrosion can lead to the release of metal ions and other compounds into the surrounding environment, potentially affecting soil and water quality. This is particularly concerning in applications where HOCl is used in large quantities or in sensitive ecosystems.
The pH of HOCl solutions is another factor that influences its environmental impact. While HOCl is most effective as a disinfectant at a slightly acidic pH, prolonged exposure to acidic conditions can accelerate corrosion and potentially harm aquatic life if released into water bodies. Proper pH control and neutralization procedures are crucial to mitigate these risks.
It is worth noting that the environmental impact of HOCl's corrosion potential varies depending on the specific surfaces and materials involved. Some materials may be more resistant to HOCl-induced corrosion, while others may degrade more rapidly. This variability underscores the importance of conducting thorough material compatibility studies and implementing appropriate protective measures to minimize environmental risks.
In conclusion, while HOCl offers several environmental benefits as a disinfectant, its corrosion potential must be carefully managed to prevent unintended ecological consequences. Balancing the need for effective sanitation with environmental protection requires ongoing research, proper application techniques, and the development of corrosion-resistant materials suitable for use with HOCl.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!