Methods and compositions for treating mitochondrial disease or disorders and heteroplasmy
a mitochondrial disease or disorder and composition technology, applied in the field of compositions, can solve the problems of high morbidity and mortality, difficult management and treatment of patients with mitochondrial disease or disorders, and considerable morbidity, so as to reduce endogenous mtdna copy number, reduce mitochondrial mass, and reduce endogenous mitochondrial function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0348]The examples in this section are offered by way of illustration, and not by way of limitation. The following examples are presented as exemplary embodiments of the invention. They should not be construed as limiting the broad scope of the invention.
example i
ion of the MirC Protocol Revealed that XbaI Degraded mtDNA In Vitro and the MTS Expression Vector Targeted Mitochondria
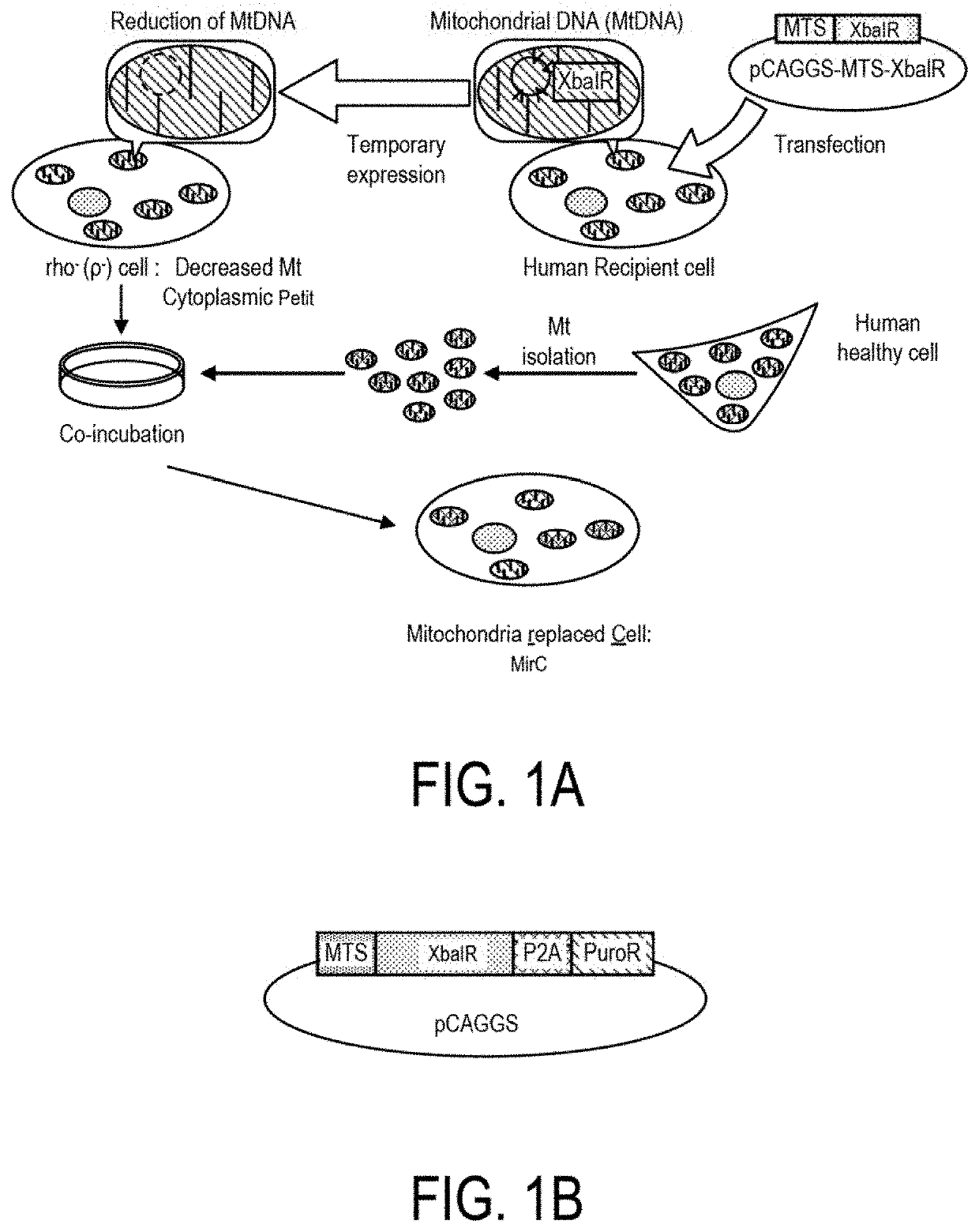
[0349]A scheme of the method used to generate a mitochondria replaced cell (MirC) is provided in FIG. 1A. First, the mammalian expression vector used to express the XbaI restriction enzyme fused to a mitochondrial-targeted sequence (MTS) was engineered by cloning the MTS-XbaI sequence into the pCAGGS vector using standard techniques known in the art (FIG. 1B). Among mitochondrial transfer signals (MTS) being reported we utilized the ND4 signal sequence in this study. The resultant expression vector also contained the puromycin resistance gene to allow for selection (FIG. 1B).
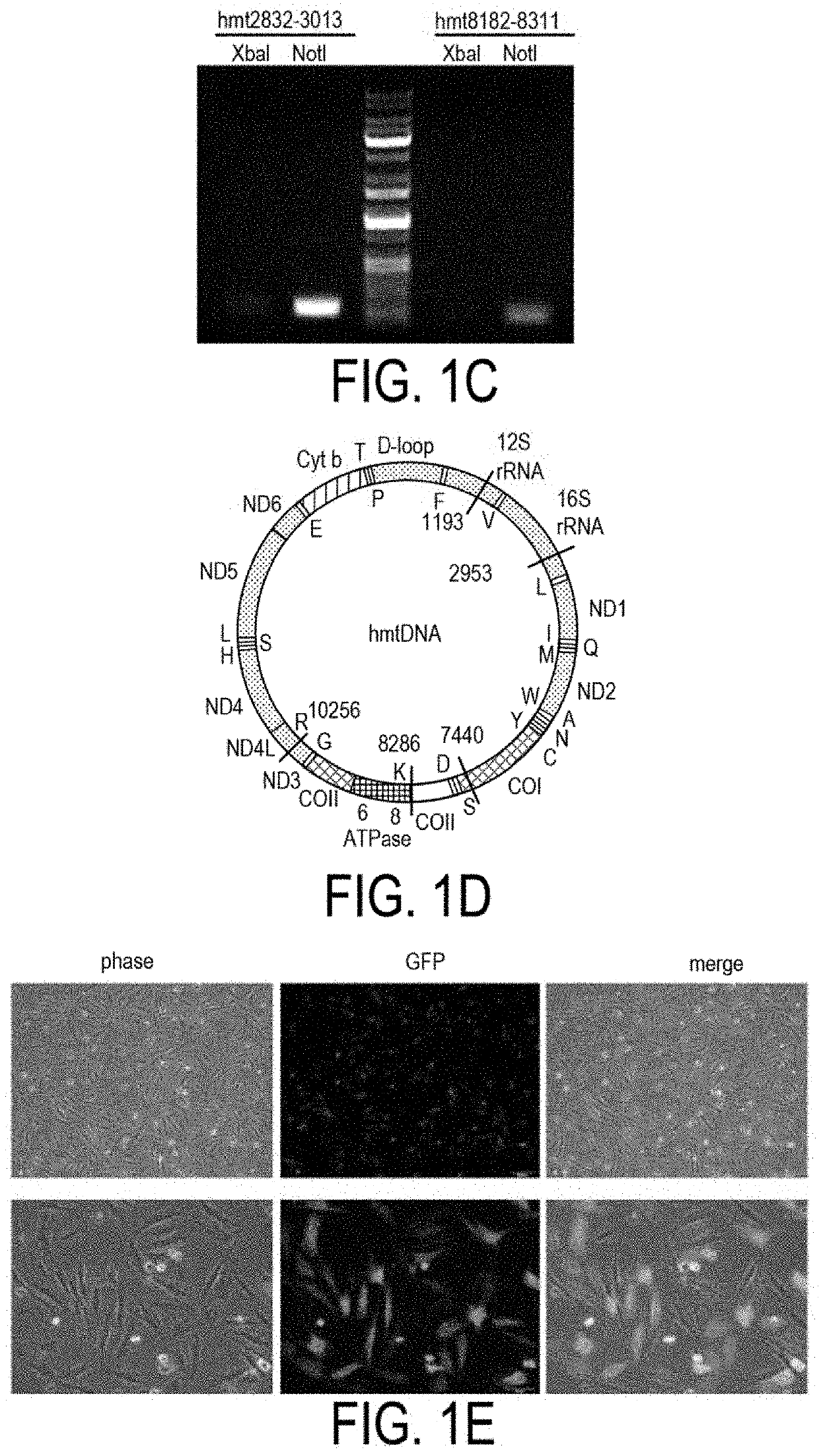
[0350]XbaIR is one of the most powerful endonucleases and a standard sequence of mtDNA named under Cambridge reference sequence (CRS) in human mitochondria genome has as many as five recognition sites targeted by the particular endonuclease (FIG. 1D). It was verified by an in vitro endonuclease ...
example ii
se MTS-XbaIR Treatment Exhibits Improved Degradation of mtDNA Relative to the Conventional Method of EtBr
[0354]The efficiency and efficacy of the MTS-XbaIR expression vector relative to the conventional method that employs ethidium bromide (EtBr) was evaluated according to the scheme illustrated in FIG. 2A. The placental venous endothelium-derived cell line EPC100 with DsRed labeled mitochondria were cultured in pyruvate-free DMEM (Wako cat #044-29765) with 10% fetal bovine serum (FBS) and 1% penicillin / streptomycin (P / S), and at day 0 the cells were either untreated (“normal”), transfected with the MTS-XbaIR expression vector (“MTS-XbaIR”), or treated with 50 ng / mL of EtBr. At day 1, the cells were cultured in DMEM with 10% FBS, and 1% P / S supplemented with 100 μg / mL pyruvate and 50 μg / mL uridine. Quantitative polymerase chain reaction (qPCR) was performed according to methods known in the art at days 3 and 5 to measure the mtDNA relative to the housekeeping gene, (3-actin (Actb). ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com