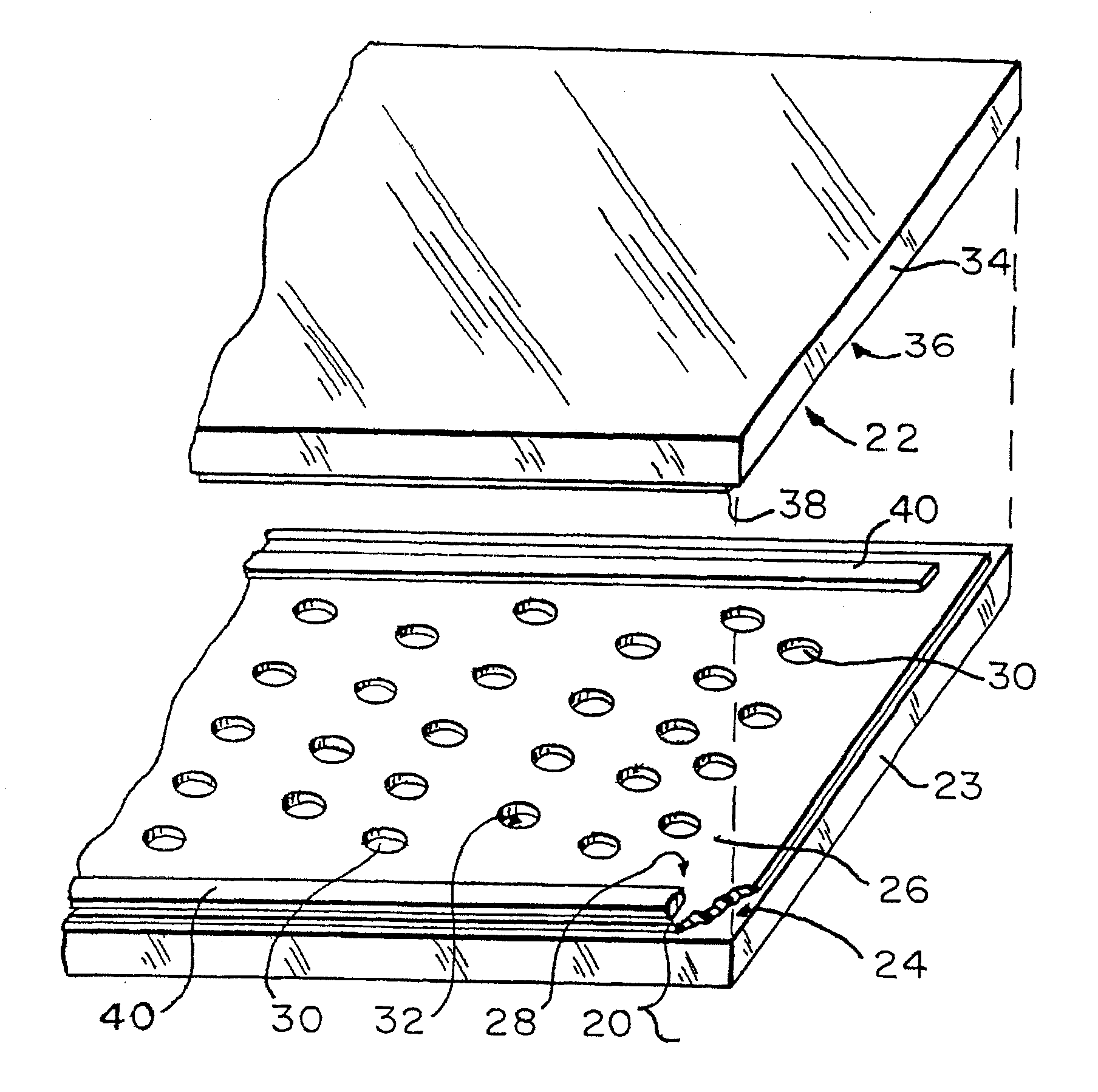
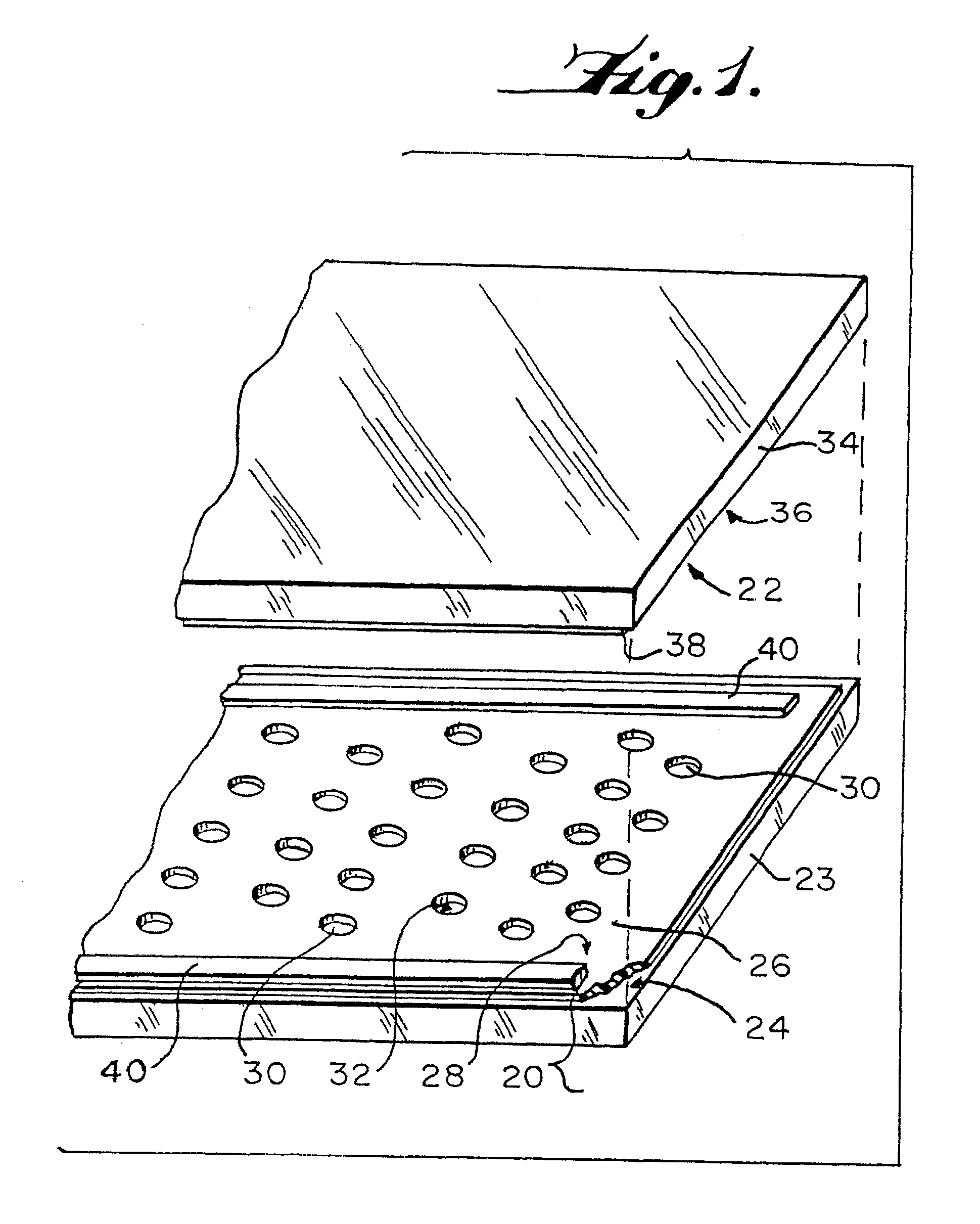
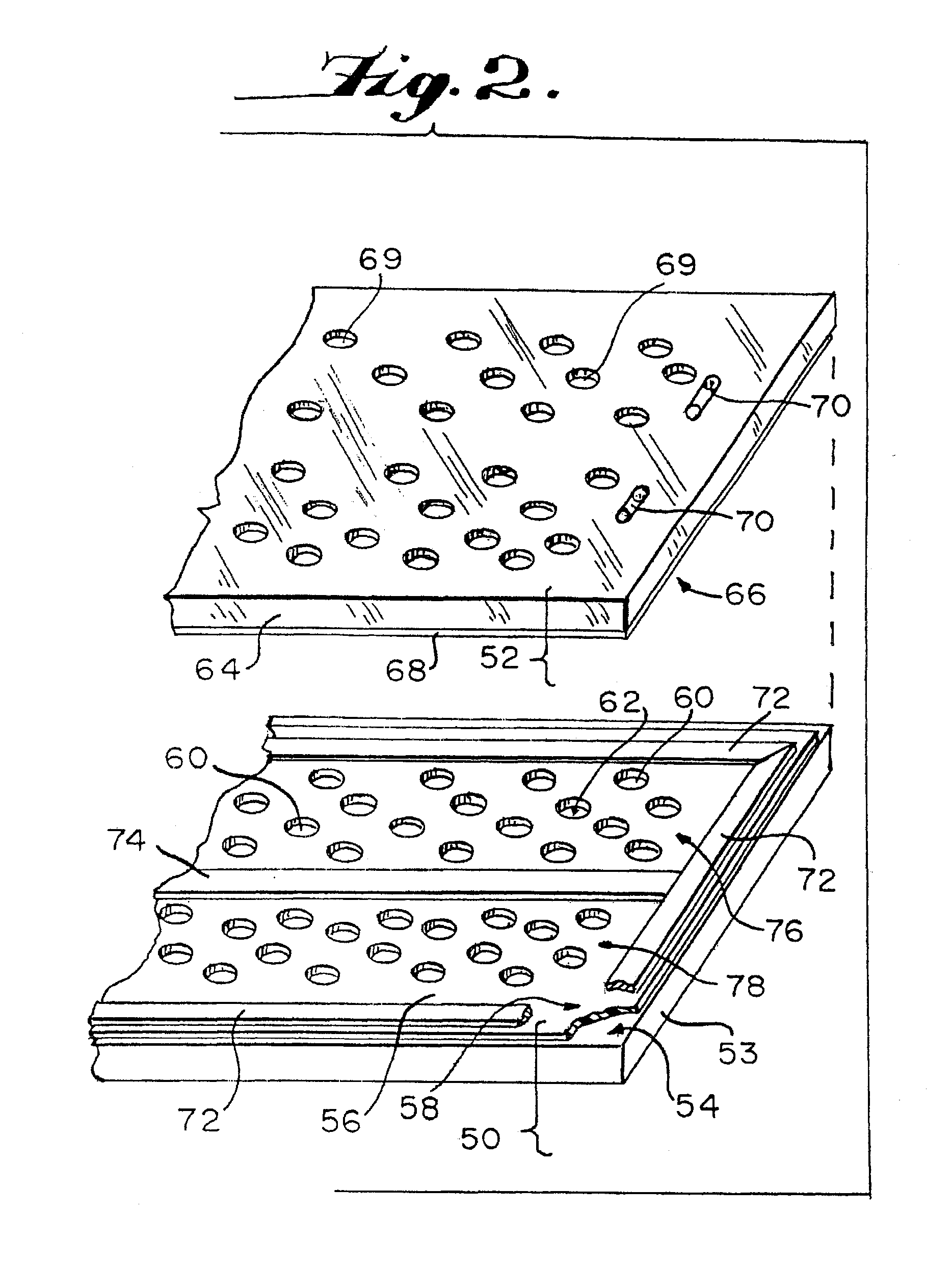
Method and device for detecting the presence of a single target nucleic acid in a sample
a nucleic acid and single target technology, applied in the field of in vitro amplification of a segment of nucleic acid, can solve the problems of pcr susceptibility, slow development, and the application of pcr to clinical diagnostics, and achieve the effect of quick and inexpensive detection of a single target nucleic acid molecul
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
AND CONTROL 1
[0147]Conventional PCR was performed in 0.2 ml polypropylene ependorf tubes to set standards. Microcapillary PCR was then performed according to the present invention on the same sample material in quartz glass microcapillaries. The PCR sample containing the nucleic acid sequence to be amplified was prepared and included materials from a “TaqMan” kit available from Perkin-Elmer, Applied Biosystems Division, Foster City, Calif. The kit contained human DNA at 10 ng / μl, the forward primer 5′-TCACCCACACTGTGCCCATCTACGA-3′ (SEQUENCE ID NO:1) and the reverse primer 5′-CAGCGGAACCGCTCATTGCCAATGG-3′ (SEQUENCE ID NO:2) that amplify a 295 bp segment of the human β actin gene, and a dual fluor-labeled probe comprising 5′-[6FAM]-ATGCCC-[TAMRA]-CCCCCATGCCATCCTGCGT-3′ (SEQUENCE ID NO:3) that is complementary to bases 31, to 56 of the PCR product. The designation FAM represents 6-carboxyfluorescein, and TAMRA represents 6-carboxytetramethyl-rhodamine. With reference to the Control 1 and...
example 2
[0165]Similar results were obtained with another preparation of human genomic DNA obtained from Promega: at 8 haploid genome equivalents (24 pg) per capillary, 4 of 4 capillaries gave maximum F / R intensity ratios≧1; at 0.7 haploid genome equivalents (2 pg) per capillary, 3 of 4 capillaries were positive; at 0.1 haploid genome equivalents (0.4 pg) per capillary, 0 of 4 capillaries were positive.
example 3
[0166]The inhomogeneity of F / R intensity ratio along the length of capillaries containing about 1 template molecule suggested that residual localization of degraded probe may be observed as a result of localized accumulation of PCR product. To investigate this possibility, amplifications in 2.5 cm long sections of capillaries containing about 0.5 haploid genome equivalent per capillary were performed. A plot of F / R intensity ratio along a few representative capillaries is shown in FIGS. 9-14. Some capillaries had a single peak while others had two. Two peaks indicate two areas where PCR product and degraded probe had accumulated. The half-widths of the peaks (measured at half-height) were about 3-6 mm. When capillaries were left overnight, the distributions broadened and flattened. Inhomogeneities in F / R intensity ratio were not seen when capillaries were examined before PCR, or after PCR in capillaries containing no template DNA or about 75 initial template molecules. Representativ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com