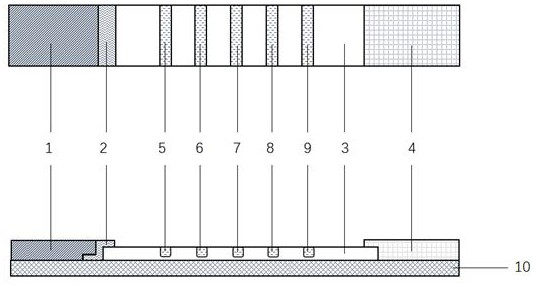
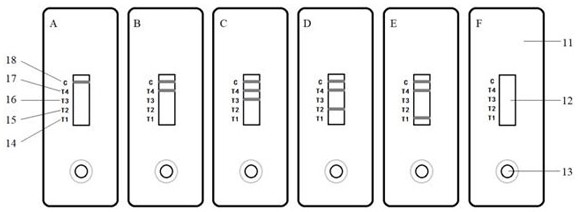
Mouse hybridoma cells and rapid diagnosis test strip for distinguishing and detecting plasmodium falciparum, plasmodium vivax, plasmodium malaria and plasmodium ovale
A technology for hybridoma cells and malaria ovale, applied in the field of bioengineering, can solve the problem of inability to effectively distinguish various pathogenic species of malaria infection, and achieve the effects of clear and intuitive result judgment, easy industrial production, and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Preparation of Plasmodium falciparum rich histone II antigen
[0051] Histidine-rich protein-II (HRP-II) is a water-soluble glycoprotein synthesized by Plasmodium falciparum in the erythroid stage. It is an ideal diagnosis because it contains large segments of amino acid repeats arranged in tandem. target molecule.
[0052] A pair of primers were designed and synthesized according to the published conserved sequence of the HRP-II gene of Plasmodium falciparum (GenBank accession number: FJ871325.1) and primer design principles:
[0053] P1: 5'-actcaagcacatgtagatgatg-3'; (shown in SEQ ID NO.1)
[0054] P2: 5'-taatggcgtaggcaatgtgtg-3'. (shown in SEQ ID NO.2)
[0055] Using anticoagulated whole blood from Plasmodium falciparum-infected patients as the template for PCR amplification: take 20 μL of whole blood and add 200 μL of red blood cell lysate (50 mmol / L NaCl, 0.015% saponin, 1 mmol / L EDTA), shake and mix well at room temperature. Set aside for 10 min to f...
Embodiment 2
[0057] Example 2 Preparation of Plasmodium falciparum-rich histone II monoclonal antibody
[0058] Immunization: Each BALB / c mouse was injected intraperitoneally for the first time with a suspension of 100 μg of the purified Plasmodium falciparum-rich histone II recombinant fusion protein + Freund's complete adjuvant purified above (Example 1), and then injected with 100 μg of purified P. falciparum adjuvant every 1 month. The suspension of recombinant fusion protein + Freund's incomplete adjuvant was used once for a total of 2 times, and the antigen was injected directly through the tail vein 3 days before the mouse was killed and the spleen was taken for cell fusion.
[0059] Generation and Screening of Hybridoma Cells The fusion of SP2 / 0 tumor cells and spleen cells of immunized mice and the cloning of hybridoma cells were performed according to conventional methods in the art. Using the purified Plasmodium falciparum histone-rich protein II recombinant fusion protein and g...
Embodiment 3
[0069] Example 3 Preparation of Plasmodium Lactate Dehydrogenase Antigen
[0070] There are 4 species of Plasmodium parasites in human body, namely Plasmodium vivax, Plasmodium malaria, Plasmodium falciparum and Plasmodium ovale. Early studies found that Plasmodium lactate dehydrogenase (LDH) and host lactate dehydrogenase are quite different in electrophoretic properties, enzymatic kinetic properties, and immunological properties. Anti-LDH antibodies are genus-specific to Plasmodium, and some anti-LDH antibodies have certain cross-reactions with lactate dehydrogenase antigens of Plasmodium from different species, and have a certain degree of cross-reaction with the three LDH isoenzymes (A4, B4) of mammals. No cross-reaction with C4). Therefore, Plasmodium lactate dehydrogenase is an ideal diagnostic target molecule.
[0071] Design and synthesize a pair of primers according to the published conserved sequence of Plasmodium LDH gene (GenBank: KX885921.1) and primer design pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com