Benzothiadiazole-TB compound as well as synthesis method and application thereof
A technology of benzothiadiazole and compounds, which is applied in the field of synthesis of benzothiadiazole-TB derivatives, can solve the problems of visible absorption and poor penetration, achieve excellent solid-state luminescence, convenient post-processing, and expand types Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
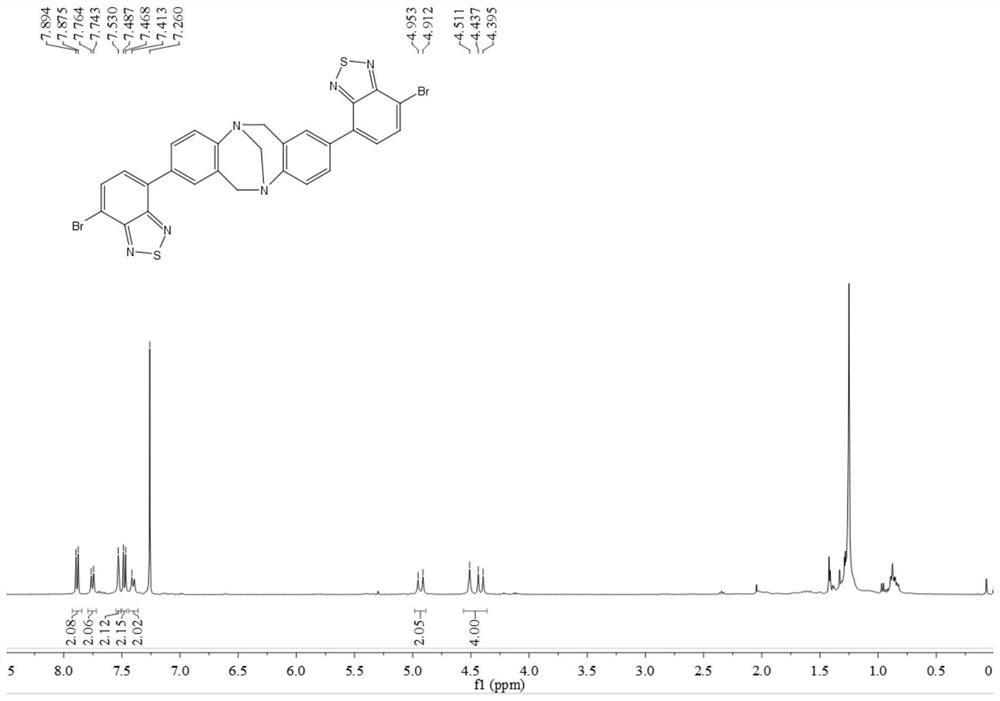
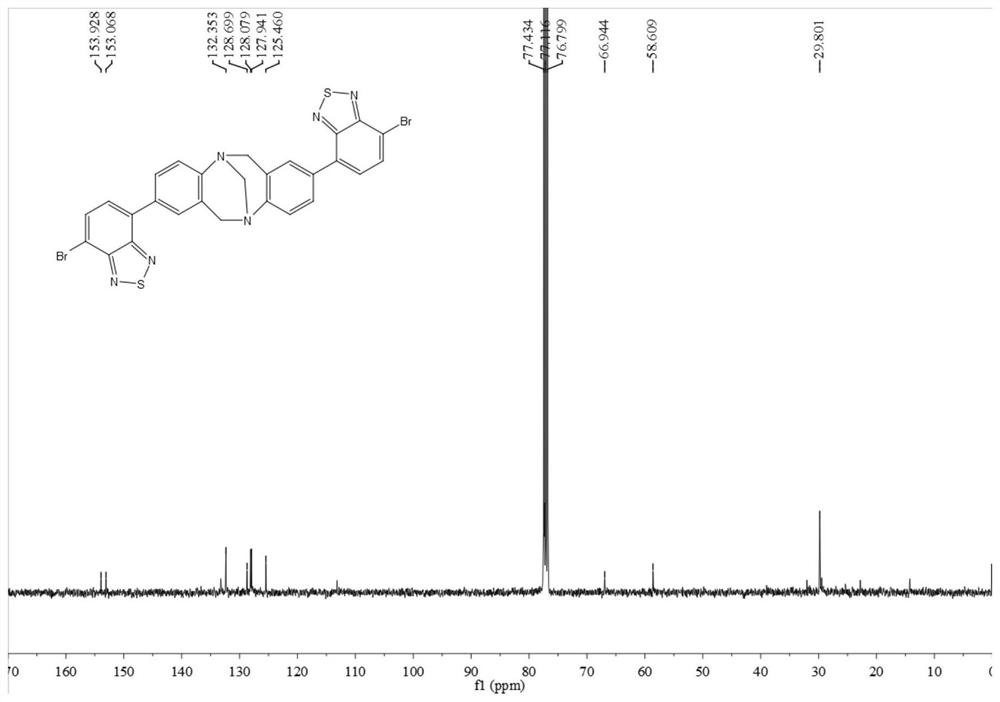
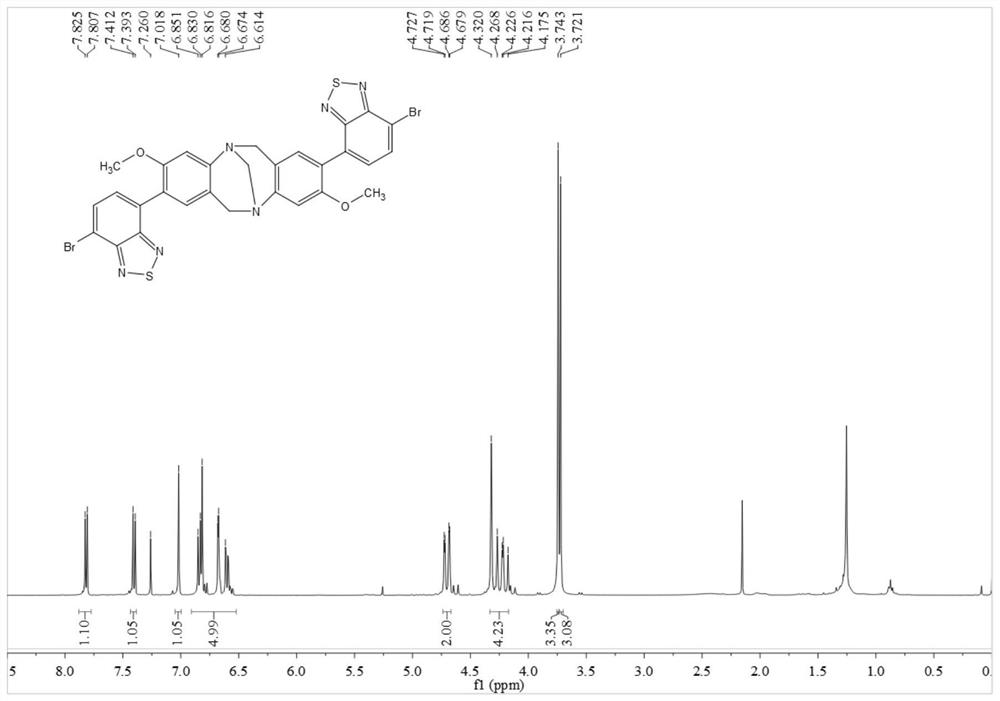
[0060]In this example, p-bromoaniline, paraformaldehyde, n-butyllithium, 4,7-dibromo-benzothiadiazole, etc. are used as raw materials to prepare through coupling reaction. Include the following steps:
[0061] Compound 1 or 2 reacts with paraformaldehyde to obtain intermediate 3 or 4, intermediate 3 or 4 reacts with trimethyl borate to obtain intermediate 5 or 6, intermediate 5 or 6 and 2,7-dibromo-benzothiophene Compound 8 or 9 can be obtained by coupling reaction of oxadiazole (7), and compound 10 can be obtained by further hydrolysis of 9.
[0062] By above-mentioned synthetic method, prepare the compound of following embodiment:
[0063] (1) Add 4-bromoaniline 1 (50.0mmol) and paraformaldehyde (100.0mmol) into a 200.0mL round-bottomed flask in turn, place it in a low-temperature tank and adjust the temperature to -15°C, and slowly add it dropwise into the flask while stirring After trifluoroacetic acid (100.0 mL, added dropwise in about 30 min), react at room temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com