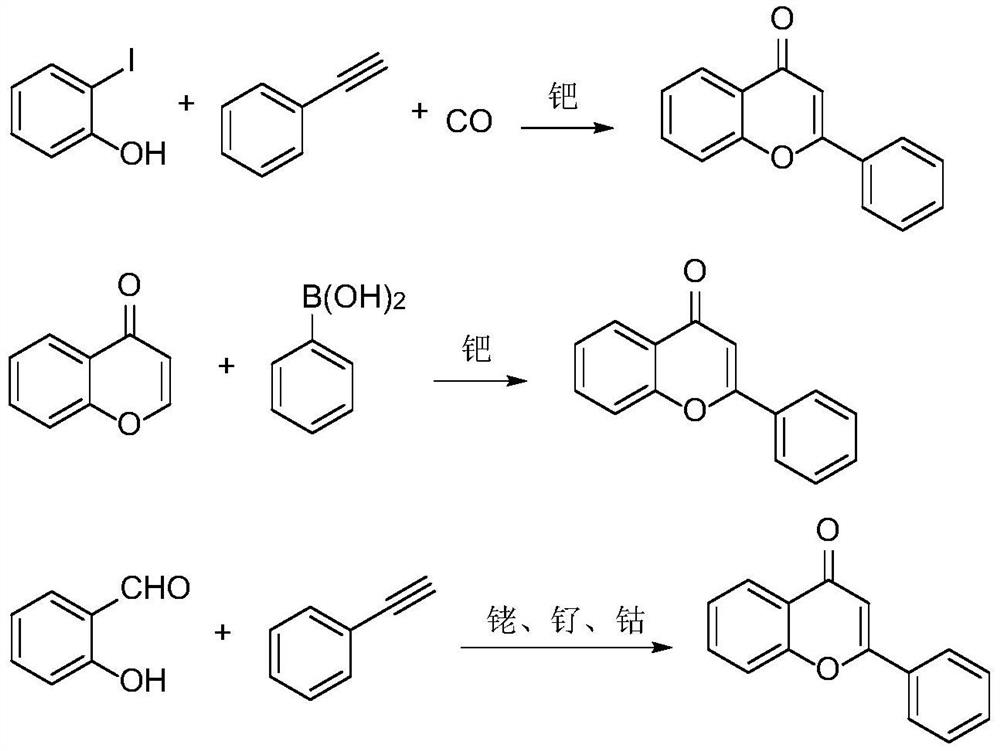
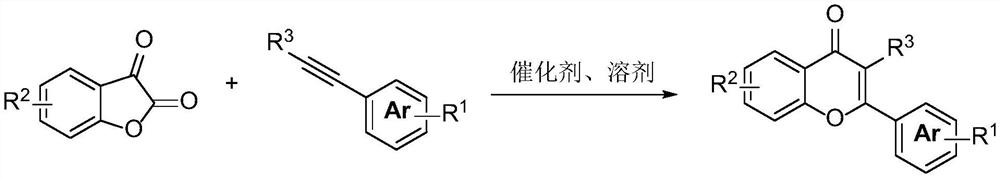
Preparation method of flavonoid compound
A technology of flavonoids and carbon atoms, applied in organic chemistry and other fields, can solve the problems of complex and expensive ligands, toxicity, and difficult to guarantee the synthesis yield, and achieve the effects of high reaction efficiency, simple operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] The synthesis of 5,7-dimethylflavone, the structural formula is as follows:
[0084]
[0085] Under nitrogen protection, add 4,6-dimethylbenzofuran-2,3-dione (0.2mmol), phenylacetylene (0.3mmol), 1,3-bis(diphenylphosphine) to the reaction flask Ethane (0.01mmol), bis(1,5-cyclooctadiene)nickel (0.01mmol), potassium acetate (0.2mmol) were dissolved in toluene (2mL). The reaction was completed at 120°C under normal pressure for 12 hours. After removing the reaction solvent, the target product 5,7-dimethylflavone was obtained by separation and purification by column chromatography: white solid, 47.6 mg, yield 95%. 1 HNMR (400MHz, CDCl 3 )δ7.91–7.82(m,2H),7.54–7.44(m,3H),7.16(s,1H),6.92(s,1H),6.67(s,1H),2.83(s,3H),2.41 (s,3H); 13 C NMR (101MHz, CDCl 3 )δ180.4, 161.3, 157.8, 143.7, 140.5, 131.8, 131.2, 129.1, 128.9, 126.0, 120.0, 115.9, 108.7, 22.6, 21.6.HRMS(ESI)m / zcalcdfor C 17 h 14 o 2 [M+Na] + 273.0883, found 273.0884.
Embodiment 2
[0086] Embodiment 2: the synthesis of 5,7-dimethylflavone
[0087] Under nitrogen protection, add 4,6-dimethylbenzofuran-2,3-dione (0.2mmol), phenylacetylene (0.3mmol), 1,3-bis(diphenylphosphine) to the reaction flask Propane (0.01 mmol), bis(1,5-cyclooctadiene)nickel (0.01 mmol), were dissolved in toluene (2 mL). The reaction was completed at 120°C under normal pressure for 12 hours. After removing the reaction solvent, the target product 5,7-dimethylflavone was obtained by separation and purification by column chromatography: white solid, 23.5 mg, yield 47%.
Embodiment 3
[0088] Embodiment 3: the synthesis of 5,7-dimethylflavone
[0089] Under nitrogen protection, add 4,6-dimethylbenzofuran-2,3-dione (0.2mmol), phenylacetylene (0.3mmol), 1,3-bis(diphenylphosphine) to the reaction flask Ethane (0.01 mmol), bis(1,5-cyclooctadiene)nickel (0.01 mmol), potassium carbonate (0.2 mmol), were dissolved in toluene (2 mL). The reaction was completed at 120°C under normal pressure for 12 hours. After removing the reaction solvent, the target product 5,7-dimethylflavone was obtained by separation and purification by column chromatography: white solid, 29.5 mg, yield 59%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com