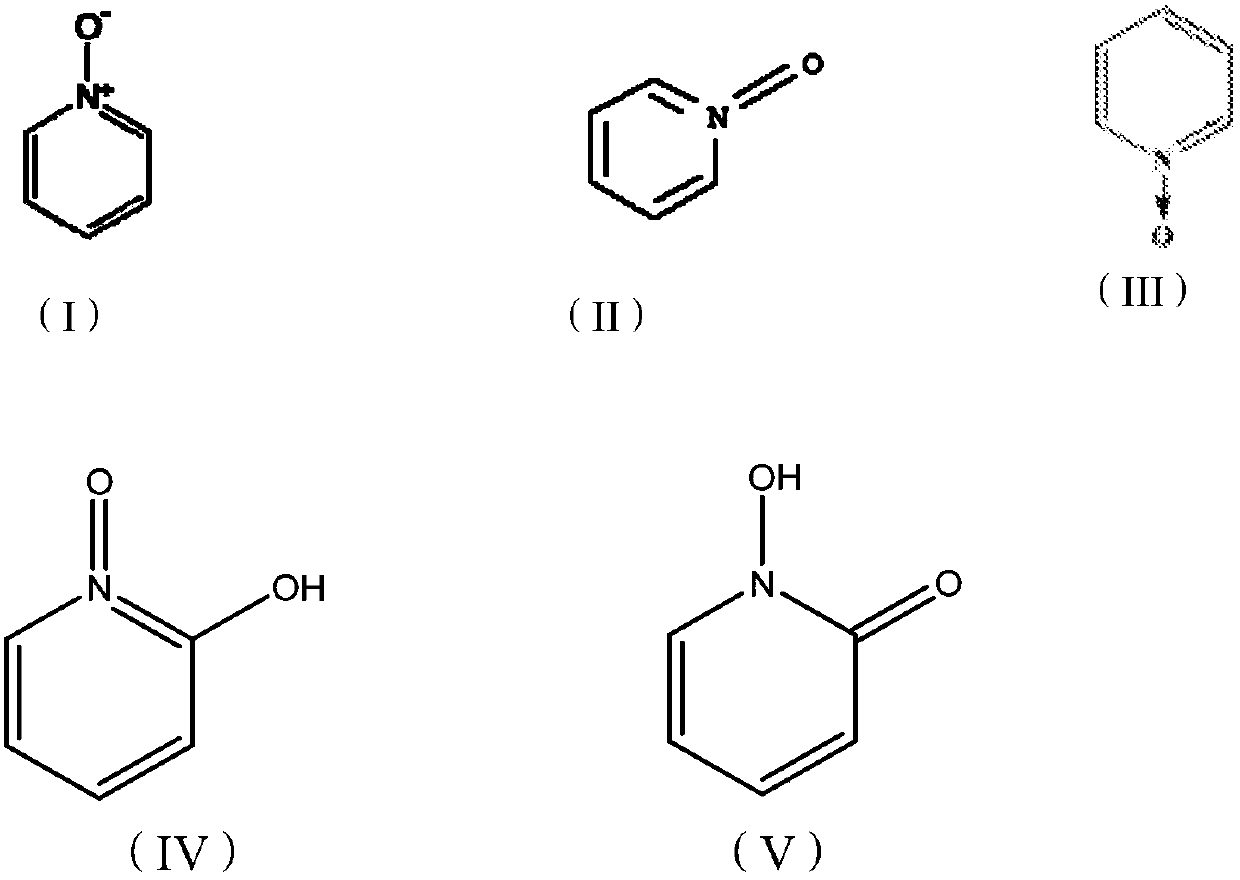
Method of treating a hair disorder with n-hydroxypyridinones
一种毛发、疾病的技术,应用在处理毛发疾病领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 4
[0179] The tonic of the present invention is prepared by conventional methods from the following exemplary components.
[0180]
[0181]
[0182] 1 Carbopol Ultrez 10, Carbopol, available from Lubrizol
[0183] 2 Arlasolve DMI, dimethylisosorbide, purchased from Croda
[0184] 3 Hydrolite-5, pentylene glycol, purchased from Symrise
[0185] 4 Klucel, hydroxypropyl cellulose, purchased from Ashland
Embodiment 5
[0187] The shampoos of the present invention are prepared by conventional methods according to the following exemplary components described in International Patent Publication WO 2008 / 027541 and US Patent Application Publication 20080059313:
[0188]
[0189]
[0190] 1 Stabileze 06, poly(methyl vinyl ether / decadiene maleic anhydride) crosspolymer, available from Ashland
[0191] 2 Laponite XLS, sodium magnesium silicate, purchased from Eckart America
[0192] 3 PrimaFlo HP22, hydroxypropyl cellulose, purchased from Ashland
[0193] 4 Ucare Polymer LR 400, polyquaternium-10, purchased from Dow
[0194] 5 Monamid DMA, Coconut Monoethanolamide, available from Croda
[0195] 6 Tegobetaine F-B, cocamidopropyl betaine, purchased from Goldschmidt Chemicals
[0196] 7 Viscasil 3000,000,: polydimethylsiloxane, purchased from Momentive
[0197] 8 Polyox WSR N-750, PEG-7M, available from Dow
[0198] 9 Kathon CG, purchased from Dow
Embodiment 6
[0200] Conditioners of the present invention are prepared by conventional methods according to the following components described in International Patent Publication WO 2008 / 027541 and US Patent Application Publication 20080059313:
[0201]
[0202]
[0203] 1 Dimethicone / Cyclomethicone: a blend of Dimethicone and Cyclomethicone with a viscosity of 18,000,000 mPas, available from GE Toshiba
[0204]2 Dimethicone Blend: Blend of Dimethicone with a viscosity of 18,000,000 mPas and Dimethicone with a viscosity of 200 mPas, available from GE Toshiba
[0205] 3 Available from GE, has a viscosity of 10,000 mPas and has the following formula (I): (R 1 ) a G3 a -Si-(-OSiG 2 ) n -(-OSiG b (R 1 ) 2-b ) m -O-SiG 3-a (Ri) a (I)
[0206] Wherein G is a methyl group; a is an integer 1; b is 0, 1 or 2, preferably 1; n is a number from about 400 to about 600; m is an integer 0; R 1 In order to meet the general formula CqH 2q A monovalent group of L, wherein q is an intege...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com