2'-C-methyl adenosine phosphoramidite monomer and synthesis method thereof
A technology of methyl adenine nucleoside phosphoramidite and synthesis method, which is applied in the field of 2'-C-methyl adenine nucleoside phosphoramidite monomer and its synthesis, and can solve the cost limitation of chemically modified RNA technology development, The phosphoramidite monomer needs to be developed to achieve the effect of simple operation, simple post-treatment, low cost and easy availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
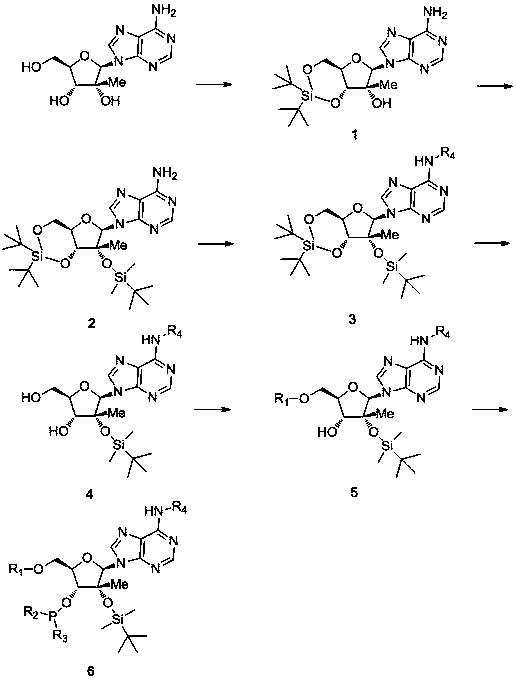
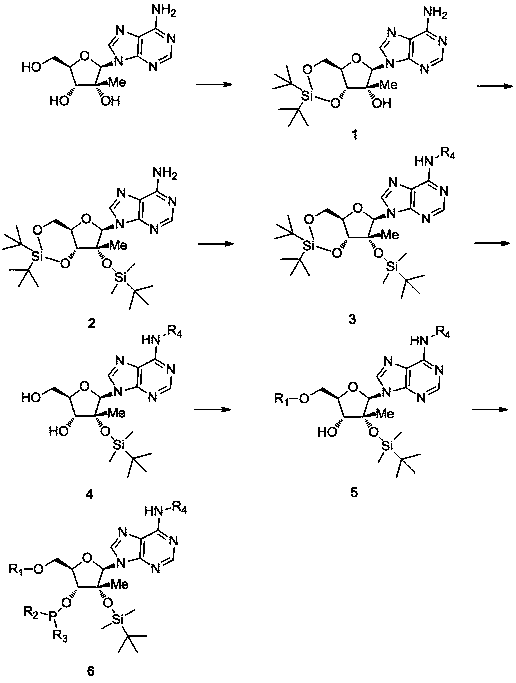
[0034] Synthesis of 3',5'-O-(di-tert-butylsilylbis)-2'-O-tert-butyldimethylsilane-2'-C-methyladenosine:
[0035]
[0036] In a 250ml two-necked bottle, add 100ml DMF and 2.8g (10mmol) 2'-C-methyladenosine (this compound has been industrialized by our company) respectively, and slowly drop 4.8g (11mmol) under the condition of nitrogen protection and 0°C ) di-tert-butylsilylbis(trifluoromethanesulfonic acid), and then stirred at 0°C for 30 minutes. Then add 3.4g (50mmol) imidazole in batches, then 25 DEG C of stirring reaction 30 minutes. 1.9 g (12 mmol) of tert-butyldimethylchlorosilane was slowly added dropwise, and then reacted with stirring at 80° C. for 3 hours. Cool to 0°C to crystallize, filter and wash with cold acetonitrile, and finally dry to obtain 4.7g 3',5'-O-(di-tert-butylsilylbis)-2'-O-tert-butyldimethylsilane- 2'-C-methyladenosine, yield 88%.
Embodiment 2
[0038] Synthesis of 3',5'-O-(di-tert-butylsilylbis)-2'-O-tert-butyldimethylsilane-N6-benzoyl-2'-C-methyladenosine:
[0039]
[0040] In a 250ml two-necked bottle, add 100ml of dichloromethane, 2.7g (5mmol) 3',5'-O-(di-tert-butylsilylbis)-2'-O-tert-butyldimethylsilane-2 '-C-methyladenosine, 1.0g (10mmol) triethylamine and 0.1g (1mmol) DMAP, under the condition of nitrogen protection and 0°C, 1.1g (7.5mmol) benzoyl chloride was added dropwise, then 25° C stirred the reaction for 3 hours. After the completion of the reaction detected by TLC, washed with water three times (50mlx3), dried with anhydrous magnesium sulfate, spin-dried the solvent, and then recrystallized with 20ml of acetonitrile to obtain 2.4g of 3',5'-O-(di-tert-butylsilylbis )-2'-O-tert-butyldimethylsilane-N6-benzoyl-2'-C-methyladenosine, the yield was 75%.
Embodiment 3
[0042] 5'-O-(4,4-dimethoxytrityl)-2'-O-[(tert-butyl)dimethylsilyl]-N6-benzoyl-2'-C-methyl Synthesis of base adenosine:
[0043]
[0044] In a 250ml two-necked bottle, 1.3g (2mmol) 3',5'-O-(di-tert-butylsilylbis)-2'-O-tert-butyldimethylsilane-N6-benzoyl-2 '-C-methyladenosine was dissolved in 5Oml of anhydrous dichloromethane. Under the conditions of ice bath and nitrogen protection, slowly add 1.0g (10mmol) pyridine hydrogen fluoride (dissolved in 6ml pyridine) dropwise, continue to stir at 0°C for 1 hour, after TLC detection reaction is complete, 50ml water and 50ml saturated sodium bicarbonate The aqueous solutions were washed once, dried with anhydrous magnesium sulfate, and the solvent was spin-dried. Then add 50ml of dichloromethane, 0.5g (5mmol) triethylamine and 0.03g (0.3mmol) DMAP respectively, and add 0.7g (2.2mmol) 4,4'-bismethyl in batches under the conditions of ice bath and nitrogen protection Oxytrityl chloride solid, then stirred at 25°C for 6 hours. Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com