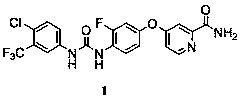
A kind of synthetic method of metafenil
A synthesis method and compound technology, which are applied in the field of synthesis of anticancer active substance metafinil, can solve problems such as being unsuitable for industrial production, and achieve the effects of mild conditions, increased yield, and reduced solvent usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
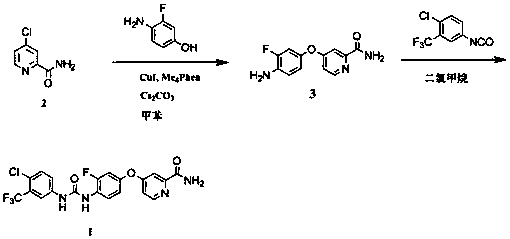
[0018] Example 1 Synthesis of 2-carbamoyl-4-((3-fluoro-4-amino)phenoxy)pyridine (compound 4)
[0019] In a dry reaction flask, put CuI (9.5g, 49.9mmoL), Cs 2 CO 3 (490.0g, 1504.0mmoL), Me 4 Phen (24.0 g, 101.6 mmoL), compound 2 (247.2 g, 1000 mmoL), toluene (500 mL). mixture under argon atmosphere at 80 - 85 0 C oil bath reaction 24h. Cool to room temperature, add ethyl acetate (1500 mL), filter the reaction solution through a silica gel column, wash the filter cake with 500 mL of ethyl acetate, combine the filtrates, and evaporate the filtrate solvent under reduced pressure. The concentrated residue was crystallized in an appropriate amount of ethyl acetate-petroleum ether to obtain 173.1 g of a white solid. The yield is 70%. 1 H NMR (400MHz, DMSO- d 6 ): δ5.21 (s, 2 H), 6.77 (dd, 1 H), 6.85 (t, 1 H), 7.01 (dd, 1 H), 7.10 (dd, 1 H), 7.34 (d, 1 H ), 7.68 (br s, 1 H), 8.10 (br s, 1 H), 8.45 (d, 1 H); MS (ESI) m / z: 248.1 (M + H + ).
Embodiment 2
[0020] Example 2 Synthesis of 4-{4-[3-(4-chloro-3-trifluoromethylphenyl)urea]-3-fluorophenoxy}pyridine-2-carboxamide (Compound 1)
[0021] Put 4-chloro-3-trifluoromethylphenylisocyanate (26.6g, 120mmoL), DMF (50mL), and N,N-diisopropylethylamine (2.6g, 20mmoL) into a reaction flask. A solution of 2-carbamoyl-4-((3-fluoro-4-amino)phenoxy)pyridine (24.7 g, 100 mmoL) in dichloromethane (350 mL) was slowly added dropwise to the reaction flask. The reaction solution was stirred at room temperature for 24 h until the reaction was complete. The solvent was evaporated. The residue was recrystallized from ethyl acetate to obtain 28.1 g of white solid (compound 1), yield 60%. 1 H NMR (400MHz, DMSO- d 6 ): δ7.18 (d, 1H), 7.20(m, 1H), 7.32(m, 1H), 7.40(d, 1H), 7.61(d,2H), 7.72(s, 1H), 8.18(m, 3H), 8.52(d, 1H), 8.73(s, 1H), 9.51(s, 1H); MS(ESI)m / z: 469.1(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com