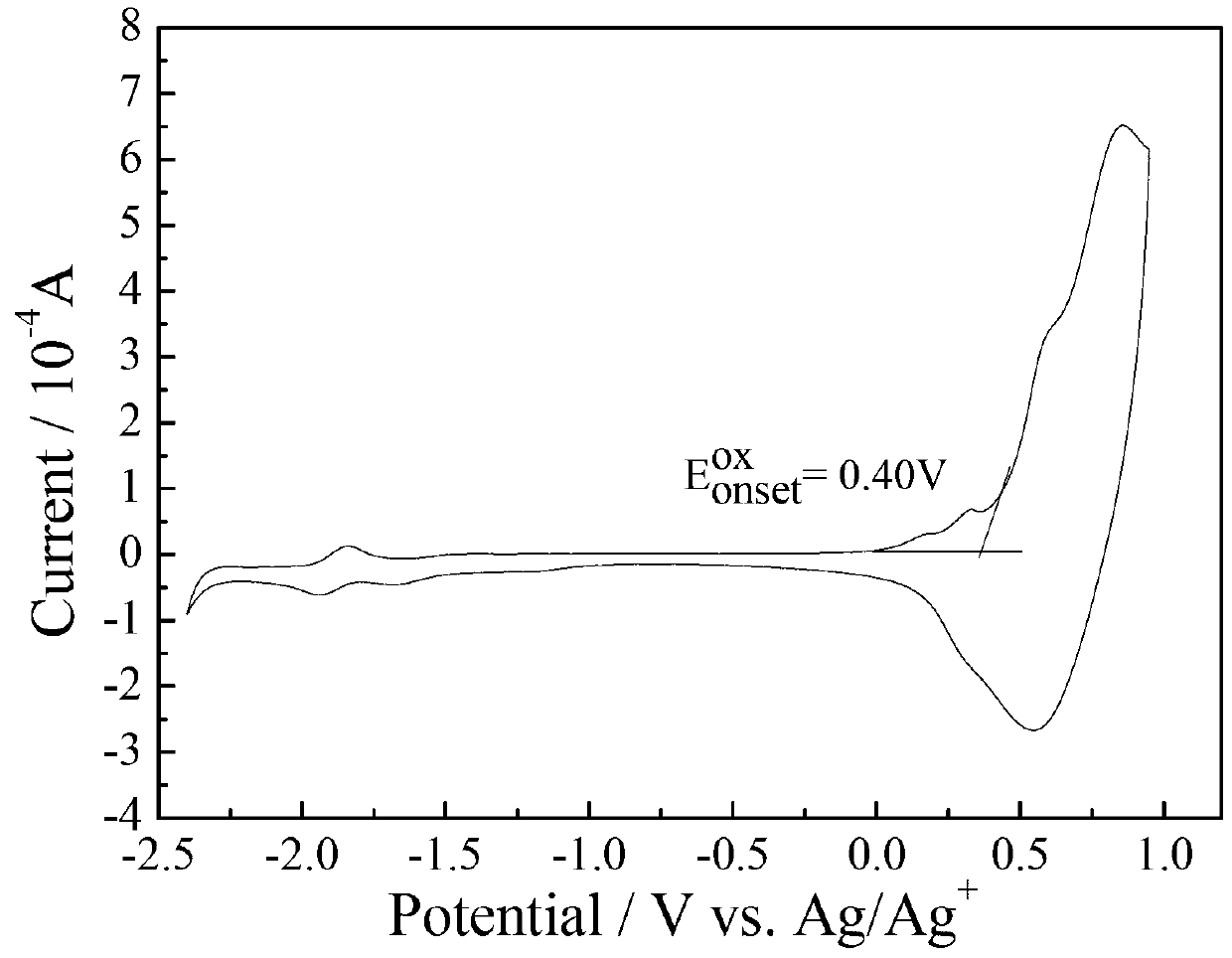
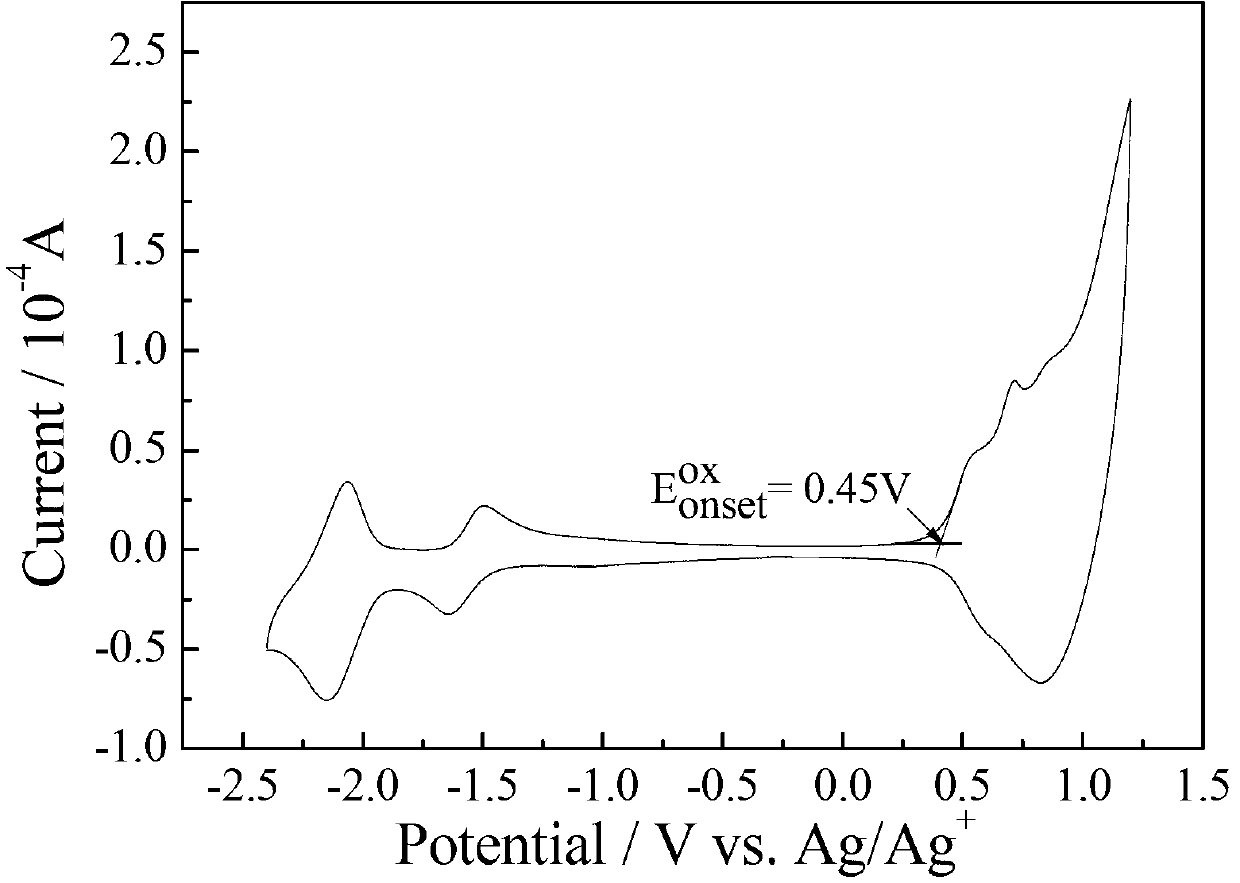
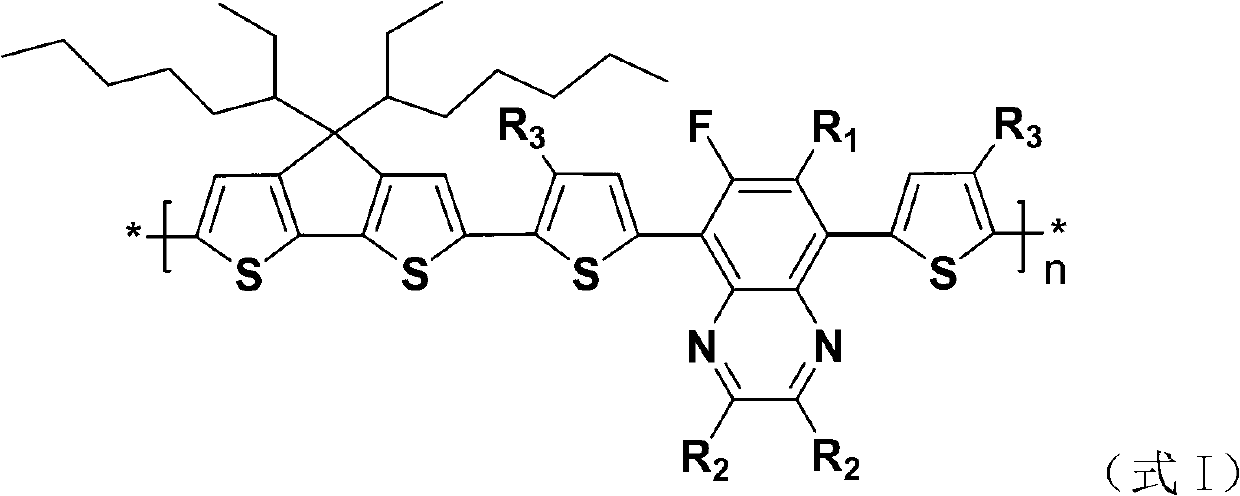
Dithiophene pentalene-fluoroquinoxaline conjugated polymer
A technology of phenocyclopentadiene and conjugated polymers, which is applied in the field of dithienocyclopentadiene-fluoroquinoxaline conjugated polymers to achieve the effect of increasing the open circuit voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of 6-fluoro-5,8-bis(5-bromo-4-hexylthiophene)-2,3-bis(3-octyloxyphenyl)quinoxaline:
[0038] (1) Preparation of 4-fluoro-3,6-dibromo-1,2-phenylenediamine (compound 1)
[0039] Follow the reaction equation shown below:
[0040]
[0041] 5-Fluoro-4,7-dibromo-2,1,3-benzothiadiazole (5g, 0.016mol) was dissolved in 150ml absolute ethanol, NaBH was added in batches at 0℃ 4 (11.1g, 0.29mol), then react at room temperature for 20h. After the reaction, concentrate to remove ethanol, add 160ml of water, extract with ethyl acetate, wash the organic phase with brine, and finally anhydrous MgSO 4 dry. Concentrate to remove the organic solvent, and the crude product obtained is purified with a silica gel column. The eluent is n-hexane / ethyl acetate (25:1, v / v) to obtain 4-fluoro-3,6-dibromo-1,2 benzene Diamine 3.5g, yield 78%.
[0042] (2) The preparation of 1,2-bis(3-octyloxyphenyl)ethanedione (compound 2) is carried out according to the reaction equation shown below:
[0043] ...
Embodiment 2
[0056] Example 2: Synthesis of 6-fluoro-5,8-bis(5-bromo-4-hexylthiophene)-2,3-bis(4-octyloxyphenyl)quinoxaline (compound 9)
[0057] Follow the reaction equation shown below.
[0058]
[0059] The synthesis method is the same as that of compound 5, except that 1,2-bis(3-octyloxyphenyl)ethanedione is replaced with 1,2-bis(4-octyloxyphenyl)ethanedione.
Embodiment 3
[0060] Example 3: Synthesis of 6,7-difluoro-5,8-bis(5-bromo-4-hexylthiophene)-2,3-bis(3-octyloxyphenyl)quinoxaline (Compound 14)
[0061] (1) Preparation of 4,5-difluoro-3,6-dibromo-1,2-phenylenediamine (compound 11)
[0062] Follow the reaction equation shown below:
[0063]
[0064] 5,6-Difluoro-4,7-dibromo-2,1,3-benzothiadiazole (10g, 0.031mol) was dissolved in 300ml absolute ethanol, and NaBH was added in batches at 0℃ 4 (22.2g, 0.59mol), then react at room temperature for 5h. After the reaction, the ethanol was removed by rotating, 200ml of water was added, ethyl acetate was added, the organic phase was washed with saturated brine, and finally anhydrous MgSO 4 dry. The crude product obtained by concentration is purified with a silica gel column, and the eluent is n-hexane / ethyl acetate (20:1, v / v) to obtain 4,5-difluoro-3,6-dibromo-1,2-benzene Diamine 6.1g, the yield is 65%.
[0065] (2) Preparation of 6,7-difluoro-2,3-bis(3-octyloxyphenyl)quinoxaline (compound 12)
[0066] Foll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com