
What is Carbolic Acid?
Carbolic acid, also known as phenol, is an organic compound with the chemical formula C6H5OH. It is a white, crystalline solid that is highly toxic and corrosive. Phenol is widely used in various industries due to its antimicrobial properties and ability to act as a precursor for other chemicals. It is commonly synthesized from coal tar or petroleum-based feedstocks through chemical synthesis processes.
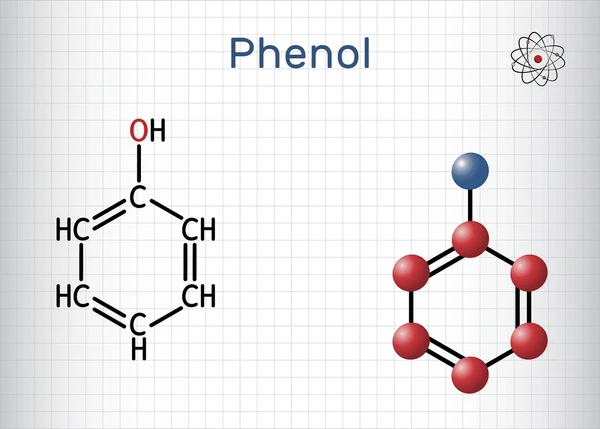
Chemical Properties of Carbolic Acid
- Aromatic Structure: Carbolic acid features a benzene ring with an attached hydroxyl group (-OH), giving it a distinct aromatic smell.
- Mild Acidity: Although termed an acid, carbolic acid is only weakly acidic in nature.
- Solubility: It exhibits moderate solubility in water but dissolves more readily in organic solvents like ethanol and ether.
- Reactivity: Carbolic acid participates in chemical reactions, including substitution, where the hydroxyl group can be replaced by other functional groups.
- Antimicrobial Properties: It acts as a potent antiseptic and disinfectant, historically used to treat wounds and prevent infections.
Historical Context
- Carbolic acid was first isolated by Friedlieb Ferdinand in 1834 through the destructive distillation of coal tar, which was a byproduct of coal gas production.
- Historically, carbolic acid was used as an antiseptic and disinfectant, and it played a significant role in the development of modern medicine. It was widely used in the late 19th century by Joseph Lister to reduce post-operative infections in surgical settings.
Health and Safety
- Acute Toxicity: Exposure to phenol can result in systemic toxicity, with symptoms including weight loss, sluggishness, muscle soreness, and weakness.
- Chronic Exposure: Long-term exposure can lead to pathological changes in various organs, including the skin, esophagus, lungs, liver, kidneys, and urogenital tract.
- Health Effects: Chronic inhalation and ingestion of phenol can cause conditions such as methemoglobinemia, haemolytic anemia, arrhythmia, pulmonary edema, and abnormal blood pressure.
- Mechanism of Toxicity: Phenols cause toxicity by disrupting cellular membranes and generating reactive oxygen species, which lead to protein denaturation and DNA damage.
- Occupational Hazards: Workers in industries like tire and rubber manufacturing face increased mortality rates from ischemic heart disease due to phenol exposure.
Synthesis and Production
- Cumene Process The most common method for producing phenol is the cumene process, which involves the oxidation of cumene (isopropylbenzene) to cumene hydroperoxide, followed by its cleavage to phenol and acetone. This process is highly efficient and is widely used in the chemical industry.
- Raschig Synthesis Another method for producing phenol is the Raschig synthesis, which involves the oxidation of benzene with nitrobenzene in the presence of sulfuric acid. This method is less common than the cumene process but is still used in some industrial applications.
- Hydrolysis of Chlorobenzene Phenol can also be produced by the hydrolysis of chlorobenzene in the presence of a catalyst. This method is less common but is still used in some chemical plants.
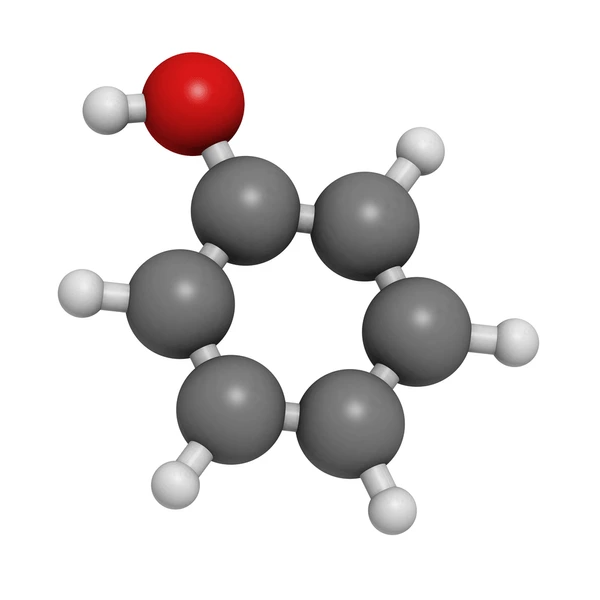
Applications of Carbolic Acid
- Disinfectant and Antiseptic: It acts as a disinfectant and antiseptic because of its antimicrobial properties. It was historically used in hospitals to control infections.
- Industrial Chemical: It is essential in producing phenolic resins, which are widely used in adhesives, coatings, and laminates.
- Pharmaceuticals: Phenol and its derivatives are utilized in antiseptic creams, ointments, and other pharmaceutical formulations.
- Chemical Synthesis: It serves as a precursor for synthesizing chemicals like aniline and aspirin.
- Water Treatment: It is used in water and sewage treatment systems to eliminate bacteria and other microorganisms effectively.
Latest Technical Innovations in Carbolic Acid
Green Chemistry Approaches
- There has been a shift towards more environmentally friendly synthesis methods. For instance, the use of catalytic oxidation processes that reduce the reliance on harmful chemicals and minimize waste6.
Biotechnological Methods
- Advances in biotechnology have led to the development of microbial fermentation processes for producing phenol and its derivatives. These methods are more sustainable and can be tailored to produce specific isomers or derivatives.
Catalytic Processes
- The use of advanced catalysts, such as heterogeneous catalysts supported on silica or other materials, has improved the efficiency and selectivity of carbolic acid production.
Process Intensification
- Innovations in process engineering have led to more efficient and compact production processes. This includes continuous flow reactors and other process intensification techniques that enhance productivity and reduce costs.
Waste Reduction Technologies
- New methods have been developed to reduce waste and by-products in the production process. This includes the recovery and reuse of by-products, and the development of closed-loop systems.
FAQs
- What is carbolic acid used for today?
It is used in the production of plastics, resins, and detergents. It’s also used in medical applications like antiseptics in low concentrations. - Why was carbolic acid used as an antiseptic in the past?
It was one of the first chemicals used to kill bacteria during surgeries, significantly reducing infection rates in the late 19th century. - Is carbolic acid safe for household use?
Not in its pure form. While diluted solutions may be found in some cleaning products, concentrated carbolic acid is highly toxic and dangerous. - What are the dangers of carbolic acid exposure?
Exposure can cause skin burns, respiratory problems, and systemic toxicity if ingested. Prolonged exposure can also affect the nervous system. - How is carbolic acid different from phenol?
Carbolic acid and phenol are the same chemical compound, with “carbolic acid” being the older term historically used in medical contexts.
To get detailed scientific explanations of carbolic acid, try Patsnap Eureka.

Learn more
Hypertonic vs. Hypotonic vs. Isotonic: What’s the Difference?
Acetophenone: A Key Compound in Fragrance and Industry
Magnesium Nitrate: Key Uses, Definition, and Innovations
