
Borophene is an extraordinary two-dimensional (2D) material composed entirely of boron atoms, emerging as a potential game-changer in the field of nanomaterials. As the world continues to explore next-generation materials for energy, electronics, and medical technology, borophene has captured significant attention due to its remarkable combination of properties — ultra-lightweight, flexible, highly conductive, and mechanically strong. While materials like graphene have long dominated the conversation around 2D materials, borophene’s unique atomic structure and multifunctional properties position it as a serious competitor, possibly surpassing graphene in specific applications.
What is Borophene? Eureka Technical Q&A explains that borophene is a revolutionary 2D material made of boron atoms, known for its ultra-light weight, flexibility, and exceptional strength—unlocking future possibilities in batteries, sensors, and next-generation electronics.
This article explores what borophene is, its structure, properties, synthesis methods, potential applications, and challenges in commercialization.
What Is Borophene?
Borophene is a single-atom-thick sheet of boron atoms arranged in a two-dimensional (2D) lattice. Unlike graphene, which consists of carbon atoms in a perfect hexagonal structure, borophene is highly polymorphic — meaning its atomic structure can vary depending on the synthesis conditions, leading to multiple possible configurations.
First successfully synthesized in 2015 by scientists at Northwestern University and Argonne National Laboratory, borophene does not occur naturally like graphene. It requires precise fabrication under ultra-high vacuum conditions, typically grown on silver (Ag) surfaces using molecular beam epitaxy (MBE).
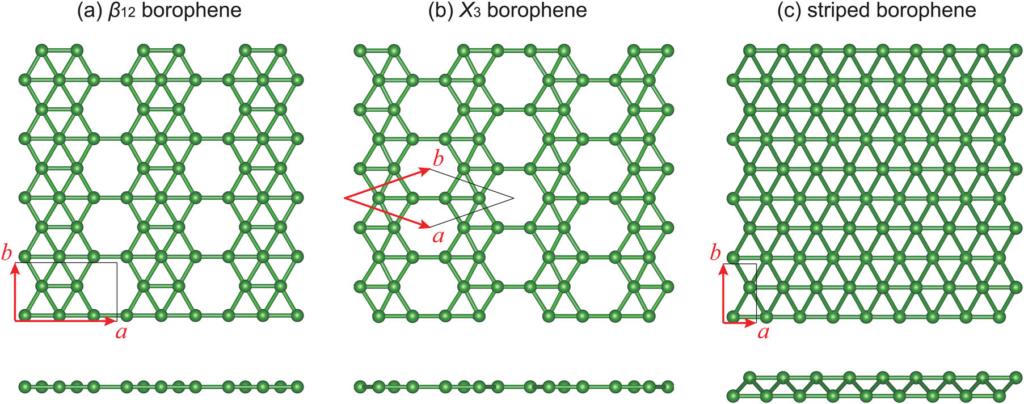
Atomic Structure of Borophene
The structure of borophene is highly dependent on the specific arrangement of boron atoms. It generally features:
- Vacancy chains or hollow hexagons integrated within a triangular lattice
- Variable patterns like β12, χ3, and other polymorphs
- Metallic bonding, providing flexibility in structure
- Anisotropic properties, meaning its characteristics vary based on direction within the material plane
This atomic versatility allows borophene to exhibit a diverse range of electronic and mechanical properties not commonly found in other 2D materials.
Unique Properties
Borophene’s combination of characteristics makes it one of the most versatile 2D materials discovered so far. These include:
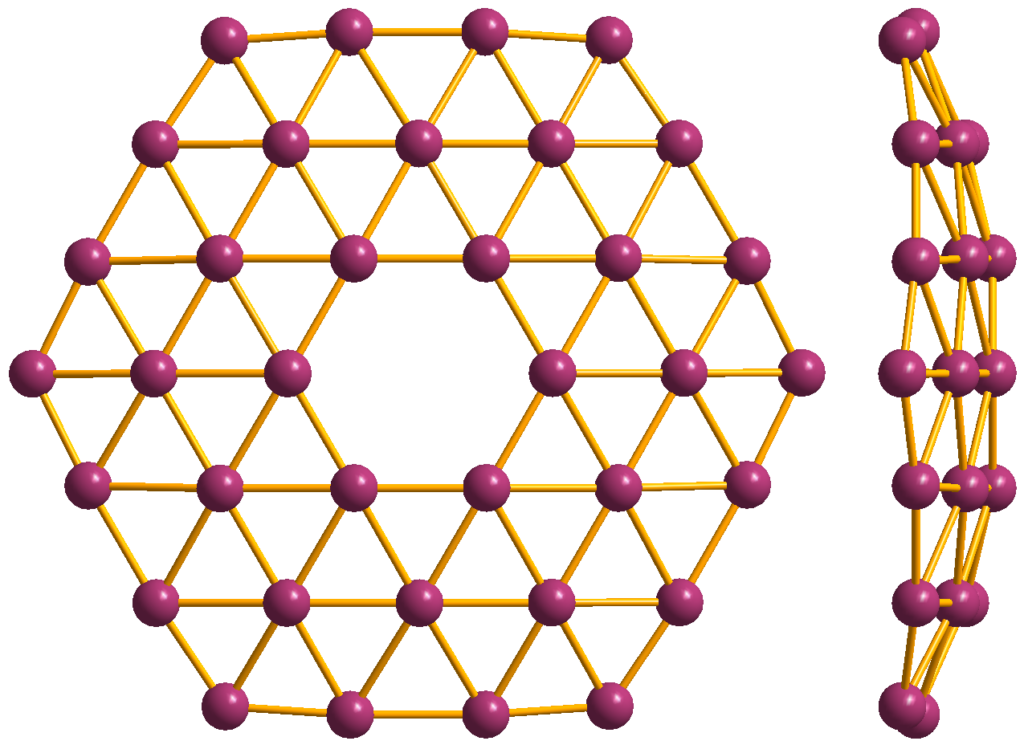
1. Ultra-Lightweight and Thin
As a single layer of boron atoms, borophene is incredibly light and thinner than most other materials, enabling its use in ultra-miniaturized devices.
2. Metallic Conductivity
Unlike many 2D materials that are semiconductors, borophene displays metallic or even superconducting behavior, depending on its structure, making it suitable for flexible electronics and high-speed devices.
3. Exceptional Flexibility and Strength
Borophene is highly flexible, with excellent mechanical strength and stretchability, outperforming even graphene in certain configurations.
4. High Anisotropic Properties
Its electrical and mechanical properties vary with direction, offering tunable features for specialized applications.
5. Superior Chemical Reactivity
Borophene is highly reactive, allowing strong interaction with other atoms or molecules, which is valuable in catalysis, energy storage, and sensor technologies.
Methods of Synthesis
Synthesizing borophene is complex and requires controlled conditions, typically involving:
Molecular Beam Epitaxy (MBE)
- Most common method
- Boron atoms are deposited onto a silver (Ag) substrate at controlled temperatures under ultra-high vacuum.
Chemical Vapor Deposition (CVD)
- An emerging method for scalable production
- Still in the early research phase for borophene synthesis
Substrate-Dependent Growth
- The structure of borophene depends heavily on the type of metal substrate used, with silver and gold being the most common choices to date.
Potential Applications
Given its exceptional properties, borophene has promising applications across various advanced technologies, some of which are still in the research or prototype phase.
1. Energy Storage and Batteries
Borophene’s high surface area and conductivity make it suitable for use in lithium-ion, sodium-ion, and other next-generation batteries, offering faster charging and higher capacity.
2. Flexible and Wearable Electronics
Its strength, flexibility, and conductivity are perfect for foldable screens, flexible circuits, and wearable sensors.
3. Hydrogen Storage
Borophene shows excellent hydrogen adsorption capability, making it a candidate material for hydrogen fuel storage systems.
4. Supercapacitors
Due to its ultra-thin structure and high conductivity, borophene can serve as a promising electrode material for high-performance supercapacitors.
5. Catalysis
Borophene’s chemical reactivity and large surface area offer potential in catalysis for fuel cells and chemical production.
6. Biomedical Applications
As research progresses, borophene may find roles in drug delivery systems, biosensors, and antimicrobial coatings.
Challenges and Limitations
Despite its incredible promise, borophene faces significant challenges before achieving widespread commercial use.
- Instability in Air: Borophene rapidly oxidizes in ambient conditions, requiring protective coatings or inert environments.
- Difficult and Costly Synthesis: MBE is expensive and limited to laboratory-scale production.
- Structural Variability: Polymorphism, while useful, also makes standardization difficult for mass production.
- Lack of Large-Scale Production Methods: Current methods are not yet industrially scalable like graphene production.
Overcoming these challenges will require advancements in synthesis techniques, stabilization strategies, and cost reduction efforts.
Borophene vs. Graphene: How Do They Compare?
Structural Differences:
- Graphene: Made of carbon atoms arranged in a hexagonal honeycomb lattice. It has been extensively studied since its isolation in 2004 and is known for its exceptional mechanical, electrical, and thermal properties.
- Borophene: Made of boron atoms, also arranged in a 2D sheet with a hexagonal structure. It was first synthesized in 2015 and has shown to have unique properties compared to graphene.
Physical Properties:
- Graphene:
- Tensile strength: ~130 GPa
- Young’s modulus: ~1 TPa
- Electrical conductivity: Up to 6000 S/cm
- Thermal conductivity: ~5000 W/mK
- Borophene:
- Tensile strength: Higher than graphene
- Young’s modulus: Higher than graphene
- Electrical conductivity: Higher than graphene
- Thermal conductivity: Lower than graphene, but still significant
Mechanical Properties:
- Both materials are extremely strong and flexible, but borophene has been shown to be more flexible and stronger than graphene. This makes borophene potentially more suitable for applications that require high flexibility and strength.
Electrical Properties:
- Both materials have high electrical conductivity, but borophene has been shown to have higher electron mobility, which is important for applications in electronics and sensors.
Thermal Properties:
- Graphene has higher thermal conductivity compared to borophene, but borophene still maintains significant thermal conductivity, making it suitable for thermal management applications.
Applications:
- Graphene: Widely used in electronics, sensors, energy storage, and biomedical applications. Its high electrical and thermal conductivity make it suitable for applications such as transparent conductive films and heat spreaders.
- Borophene: Still an emerging material, but its unique properties make it promising for applications in flexible electronics, sensors, and catalytic chemistry. Its higher strength and flexibility compared to graphene make it suitable for applications that require these properties.
| Property | Borophene | Graphene |
|---|---|---|
| Composition | Boron atoms | Carbon atoms |
| Conductivity | Metallic, sometimes superconducting | High electrical conductivity (semi-metal) |
| Strength | High, flexible, anisotropic | Extremely high tensile strength |
| Stability | Less stable in air | Very stable under ambient conditions |
| Applications | Energy storage, hydrogen storage, catalysis, electronics | Electronics, sensors, composites, batteries |
While graphene remains unmatched in certain areas like stability and mechanical strength, borophene’s superior flexibility, metallic conductivity, and chemical reactivity offer new possibilities that graphene cannot easily achieve.
Future Outlook of Borophene Research
Borophene research is still in its early stages compared to graphene, but its potential across energy, electronics, healthcare, and materials science is driving increased investment and scientific interest.
Future breakthroughs in borophene synthesis, stabilization, and functionalization could pave the way for:
- Ultra-light batteries and supercapacitors
- Flexible, high-speed electronic devices
- Hydrogen-powered energy systems
- Smart wearable biomedical devices
- Next-generation catalysts and filters
If these challenges are overcome, borophene could very well be at the heart of future technologies, alongside or even surpassing graphene in specific applications.
Conclusion
Borophene represents a new frontier in the world of ultra-light, high-performance 2D materials. With exceptional electrical, mechanical, and chemical properties, it has the potential to revolutionize a wide range of industries, from energy storage and flexible electronics to biomedical applications and catalysis.
However, for borophene to transition from laboratory wonder to commercial reality, significant research is still needed in scalable production methods, air stability, and integration into functional devices. Still, its unique capabilities have already positioned borophene as a key player in the future of materials science.
FAQs
Borophene can be more flexible and adaptable than graphene, though graphene generally has superior tensile strength.
Potential uses include batteries, supercapacitors, hydrogen storage, flexible electronics, and catalysis.
Primarily through molecular beam epitaxy (MBE) on silver substrates under ultra-high vacuum conditions.
It combines metallic conductivity, ultra-light weight, flexibility, and chemical reactivity in a 2D material.
Not entirely, but borophene may outperform graphene in specific areas like hydrogen storage, energy storage, and flexible electronics.
To get detailed scientific explanations of borophene, try Patsnap Eureka.


