
Electrolytic cells are vital devices in electrochemistry, used to drive non-spontaneous chemical reactions using an external power source. They play a crucial role in many industrial processes, from metal refining to electroplating and water splitting. Understanding how electrolytic cells work involves exploring their components, the role of electrodes, chemical reactions, and real-world applications.
This article provides a complete guide to electrolytic cells, explaining their working principle, key parts, reaction mechanisms, and common uses in science and industry.
How do electrolytic cells work? Eureka Technical Q&A explains how electrolytic cells use electrical energy to drive non-spontaneous chemical reactions, highlighting the role of electrodes, ion movement, and their applications in metal plating, electrolysis, and industrial processing.
What Is an Electrolytic Cell?
An electrolytic cell is an electrochemical device that uses electrical energy to drive a chemical reaction that would not occur spontaneously. Unlike galvanic (or voltaic) cells, which produce electricity from spontaneous redox reactions, electrolytic cells require an external power source, like a battery or power supply, to force the reaction to proceed.
Key Principle:
→ Electricity in → Chemical reaction out
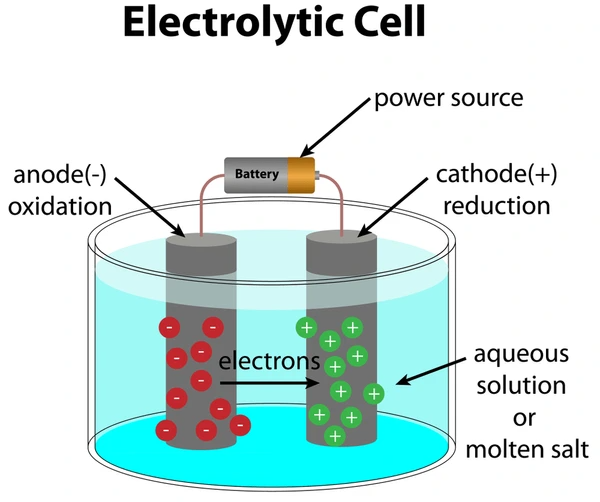
Basic Structure of an Electrolytic Cell
An electrolytic cell consists of three essential components:
1. Electrolyte
A solution or molten ionic compound that allows ions to move and complete the circuit.
2. Electrodes
Two solid conductors submerged in the electrolyte:
- Cathode: The electrode where reduction (gain of electrons) occurs.
- Anode: The electrode where oxidation (loss of electrons) occurs.
3. External Power Source
Provides the energy needed to drive the non-spontaneous redox reaction by pushing electrons through the circuit from the anode to the cathode.
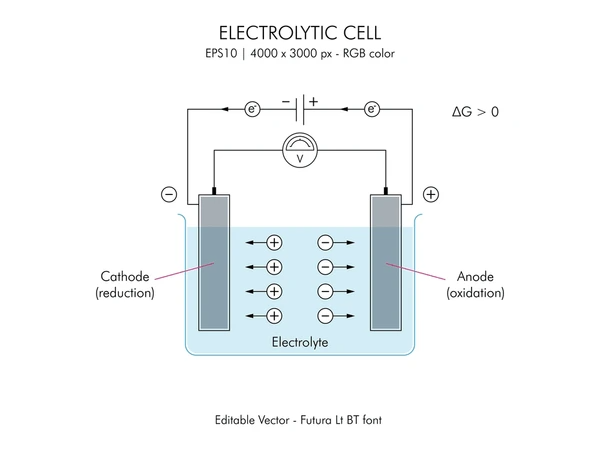
How Electrolytic Cells Work: Step-by-Step Process
1. Power Supply Activation
An external voltage source is connected to the electrodes:
- Positive terminal → Anode
- Negative terminal → Cathode
2. Movement of Electrons
- Electrons flow from the anode to the cathode through the external circuit.
3. Oxidation at the Anode
- The anode loses electrons (oxidation), releasing metal ions or forming gases.
4. Reduction at the Cathode
- The cathode gains electrons (reduction), depositing metals or forming new substances.
5. Ion Migration in Electrolyte
- Positive ions (cations) move toward the cathode.
- Negative ions (anions) move toward the anode.
Example: Electrolysis of Molten NaCl (Sodium Chloride)
Electrodes:
- Cathode (-): Sodium ions (Na⁺) gain electrons → Sodium metal (Na) forms.
- Anode (+): Chloride ions (Cl⁻) lose electrons → Chlorine gas (Cl₂) forms.
Half-Reactions:
Cathode (Reduction): Na⁺ + e⁻ → Na (s)
Anode (Oxidation): 2Cl⁻ → Cl₂ (g) + 2e⁻
Overall Reaction:
2NaCl (l) → 2Na (s) + Cl₂ (g)
Electrodes in Electrolytic Cells: Key Roles
| Electrode | Reaction | Charge | Movement |
|---|---|---|---|
| Cathode | Reduction (gain of electrons) | Negative terminal | Cations move here |
| Anode | Oxidation (loss of electrons) | Positive terminal | Anions move here |
Comparison: Electrolytic Cell vs. Galvanic Cell
- Function and Purpose:
- Galvanic Cell: This type of cell generates electric current through spontaneous redox (reduction-oxidation) reactions. It essentially acts as a battery, converting chemical energy into electrical energy. Common examples include alkaline batteries and lithium-ion batteries used in portable devices.
- Electrolytic Cell: In contrast, an electrolytic cell requires an external source of electrical energy to drive a non-spontaneous redox reaction. It is used for electrolysis, where electrical energy is used to drive chemical reactions, such as the decomposition of water to produce hydrogen and oxygen.
- Components:
- Both types of cells have similar basic components: electrodes (anode and cathode), electrolyte, and separators. However, the arrangement and materials can differ based on the specific application and type of cell.
- Applications:
- Galvanic Cells: Widely used in portable electronics, electric vehicles, and other applications requiring a reliable power source.
- Electrolytic Cells: Used in industrial processes such as the production of hydrogen, electroplating, and in processes like the chlor-alkali process for producing chlorine and sodium hydroxide.
- Efficiency and Energy Conversion:
- Galvanic cells are designed to efficiently convert chemical energy to electrical energy with minimal energy loss. Electrolytic cells, on the other hand, aim to maximize the efficiency of electrical energy conversion into chemical energy, although this is often less efficient than the energy conversion in galvanic cells.
| Feature | Electrolytic Cell | Galvanic Cell |
|---|---|---|
| Energy Source | External power required | Generates electricity spontaneously |
| Cathode | Reduction (negative) | Reduction (positive) |
| Anode | Oxidation (positive) | Oxidation (negative) |
| Electron Flow | From anode to cathode | From anode to cathode |
| Example | Electrolysis of NaCl | Daniell Cell (Zn/Cu cell) |
Common Applications of Electrolytic Cells
Electrolytic cells have many industrial and laboratory uses, including:
1. Electroplating
- Coating metal objects with a thin layer of another metal (gold, silver, nickel) for protection or aesthetics.
2. Electrorefining of Metals
- Purifying metals like copper, zinc, and aluminum by removing impurities through electrolysis.
3. Production of Elements
- Generating pure substances like:
- Chlorine gas (Cl₂)
- Hydrogen gas (H₂)
- Sodium metal (Na)
- Aluminum (Al)
4. Electrolysis of Water
- Splitting water (H₂O) into hydrogen and oxygen gases.
Half-Reactions:
- Cathode: 2H₂O + 2e⁻ → H₂ + 2OH⁻
- Anode: 2H₂O → O₂ + 4H⁺ + 4e⁻
5. Manufacturing Batteries
- Recharging batteries like lead-acid or lithium-ion cells using electrolytic principles.
6. Cleaning and Restoration
- Using electrolysis to clean rust or corrosion from metal artifacts.
Advantages of Electrolytic Cells
- Controlled chemical production
- Purification of metals
- Environmentally friendly methods of metal extraction
- Flexible for both laboratory and industrial-scale processes
Limitations of Electrolytic Cells
- High energy consumption
- Requires careful control of conditions
- Possible release of hazardous gases (like chlorine)
- Electrode material can degrade over time
Conclusion
Electrolytic cells are essential devices in electrochemistry, transforming electrical energy into chemical change. Their ability to drive non-spontaneous reactions makes them indispensable for industrial manufacturing, metal purification, electroplating, and green energy technologies like hydrogen production.
Understanding how electrolytic cells work — from the role of electrodes to the specific chemical reactions involved — offers valuable insights into a wide range of scientific and industrial processes.
FAQs
To drive chemical reactions using electricity, especially reactions that do not occur naturally.
The anode is positive because it attracts anions and is connected to the positive terminal of the power source.
Reduction occurs — ions gain electrons and form neutral atoms or molecules.
Yes, particularly in rechargeable batteries during the charging process.
Electrolysis of water to produce hydrogen and oxygen gases or electroplating jewelry with gold.
To get detailed scientific explanations of electrolytic cell, try Patsnap Eureka.


