Overview
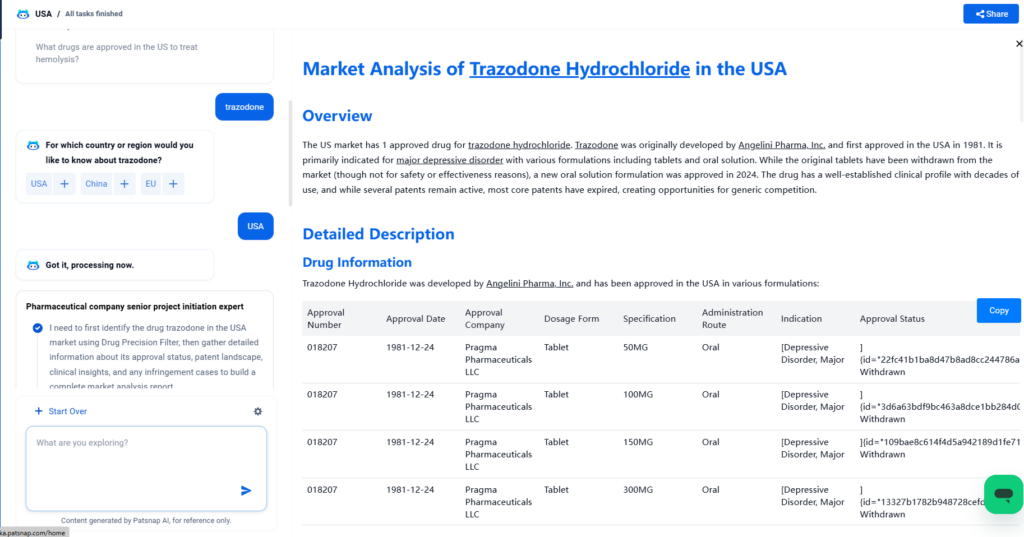
The US market has 1 approved drug for trazodone hydrochloride. Trazodone was originally developed by Angelini Pharma, Inc. and first approved in the USA in 1981. It is primarily indicated for major depressive disorder with various formulations including tablets and oral solution. While the original tablets have been withdrawn from the market (though not for safety or effectiveness reasons), a new oral solution formulation was approved in 2024. The drug has a well-established clinical profile with decades of use, and while several patents remain active, most core patents have expired, creating opportunities for generic competition.
Detailed Description
Drug Information
Trazodone Hydrochloride was developed by Angelini Pharma, Inc. and has been approved in the USA in various formulations:

Special Review
| Organization Name | Indication | Special Review | Country | Approval Time |
|---|---|---|---|---|
| Epygenix Therapeutics, Inc. | CDKL5 Deficiency Disorder | Orphan Drug | United States | 2017-08-18 |
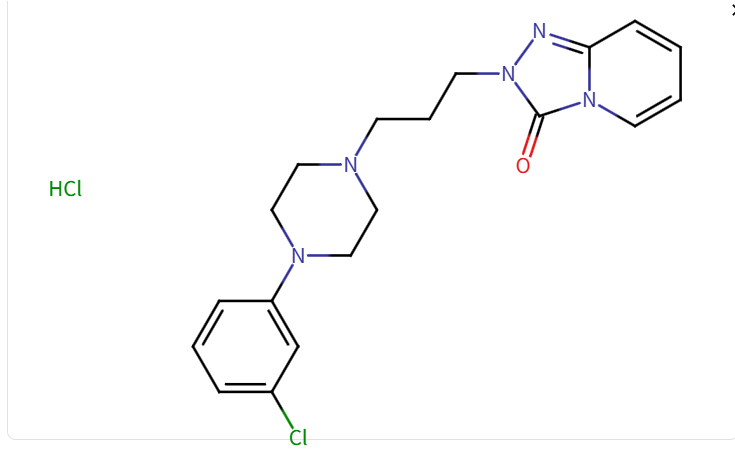
Structure
Patent Barrier Analysis
Registration Patent Analysis
FDA Orange Book patents include formulation patents from various companies with some still active:
| Patent Number | Simple Legal Status | Application Date | Estimated Expiry | Patent Type | Applicant | Source |
|---|---|---|---|---|---|---|
| US6607748B1 | Inactive | 2000-06-29 | 2020-06-29 | Formulation | LABOPHARM BARBADOS LTD | FDA Orange Book |
| US8133893B2 | Active | 2008-07-23 | 2029-03-13 | Process, Formulation | Aziende Chimiche Riunite Angelini Francesco – A.C.R.A.F. – SpA | FDA Orange Book |
| US7829120B2 | Active | 2006-09-11 | 2027-03-27 | New Use, Formulation | Angelini Pharma, Inc. | FDA Orange Book |
Other Patent Barrier Analysis
Original Patents:
| Patent Number | Simple Legal Status | Application Date | Estimated Expiry | Patent Type | Applicant |
|---|---|---|---|---|---|
| US9439866B2 | Active | 2010-09-28 | 2026-08-02 | New Use | Angelini Pharma, Inc. |
Non-Original Patents: There are numerous non-original patents related to trazodone, including formulation patents, process patents, and new use patents. Selected examples include:

Patent Statement Information
Pre-MMA patent statements exist for the trade name “Desyrel” and statements from 2010 for “Oleptro” with 180-day exclusivity status marked as “Extinguished” as of August 10, 2020.
Clinical Results
Based on FDA Label Clinical Insight, trazodone hydrochloride has been extensively studied in controlled clinical trials for major depressive disorder:
- Controlled Clinical Studies:
- Clinical trials established efficacy in treating major depressive disorder (MDD) in adults
- Formulation marketed as Raldesy was evaluated through controlled studies comparing outcomes with placebo-treated patients.
- Safety and tolerability were carefully monitored, with common adverse events (≥2% of patients and higher than placebo) systematically documented.
- Postmarketing Surveillance Studies:
- Extended safety monitoring identified less common adverse reactions (<2% incidence)
- Rare and serious adverse events were documented, including cardiac events (arrhythmias, QT prolongation), endocrine disorders (SIADH), priapism, and increased bleeding risk.
- Real-world data collection provided insights into the safety profile in broader patient populations
- Additional Experimental Evaluations:
- Dose-response studies revealed relationships between dosage and adverse effects like conduction block, orthostatic hypotension, and syncope
- Some cardiac events (ventricular tachycardia) were observed even at lower doses (100 mg per day or less).
- Laboratory parameters were monitored to detect hepatobiliary disorders like cholestasis and jaundice
Infringement Cases
No specific patent infringement incidents involving trazodone hydrochloride have been reported based on the available information.
Policy and Regulatory Risk Warning
After a comprehensive search, trazodone hydrochloride has no market exclusivity or data protection period currently active in the United States. The drug has been on the market since 1981, and any regulatory exclusivity periods have expired.
Market Entry Assessment & Recommendations
Based on the comprehensive analysis, the following recommendations can be made:
- Generic Market Opportunities:
- With most core patents expired and the original tablet formulations withdrawn (though not for safety reasons), there are opportunities for generic manufacturers to enter or expand in this market.
- The original compound patent has long expired, and many of the formulation patents have also expired or will expire soon.
- Formulation Innovation:
- Companies should consider developing novel formulations or delivery systems to differentiate their products, as evidenced by the recent approval of an oral solution in 2024.
- Extended-release formulations may still have some patent protection but offer improved patient compliance and potentially better side effect profiles.
- New Indication Development:
- The orphan drug designation for CDKL5 Deficiency Disorder suggests potential for expanding trazodone’s use beyond depression.
- Several patents cover new uses for trazodone, indicating ongoing research into additional therapeutic applications.
- Combination Products:
- Consider developing combination products with complementary medications, as suggested by some of the patents (e.g., combinations with gabapentin for pain management).
- Market Positioning:
- For innovator companies, focus on brand loyalty, physician education, and highlighting the long safety record.
- For generic entrants, emphasize cost advantages and bioequivalence.
- Regulatory Strategy:
- Monitor any changes in regulatory requirements for antidepressants.
- Ensure compliance with current FDA guidelines for psychiatric medications.
- Patient Support Programs:
- Develop comprehensive patient support programs to enhance adherence and patient outcomes.
- Consider digital health tools to monitor and improve treatment effectiveness.
The trazodone market in the USA represents a mature product with established clinical use but still offers opportunities for innovation in formulation, indication expansion, and patient-centered approaches to treatment.