
Titration is a key analytical technique used in chemistry to determine the concentration of an unknown solution using a solution of known concentration. This article will explain what titration is, explore its main types, and walk you through a complete titration procedure with examples and tips.
Need help understanding titration procedures or choosing the right indicators? Eureka Technical Q&A connects you with experienced chemists who break down titration types, techniques, and calculations—making lab work more accurate and easier to grasp, whether you’re a student or a researcher.
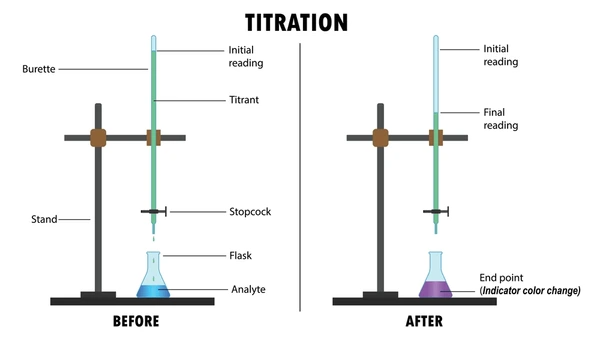
What Is Titration?
Titration is a quantitative chemical analysis method used to determine the unknown concentration of a solute in a solution. It involves the gradual addition of a titrant (a solution of known concentration) to a solution containing the analyte (unknown concentration) until the reaction reaches completion—usually signaled by a color change or pH shift.
Key Terms
- Titrant: The solution with known concentration added from a burette.
- Analyte: The solution with unknown concentration being analyzed.
- Equivalence point: The moment when the titrant has completely reacted with the analyte.
- End point: The physical change (often color) used to signal the equivalence point.
- Indicator: A chemical dye that changes color at or near the equivalence point.
Common Types
1. Acid-Base Titration
Involves an acid reacting with a base, commonly using indicators like phenolphthalein or methyl orange.
Example: HCl titrated with NaOH
2. Redox Titration
Based on oxidation-reduction reactions, using redox indicators or self-indicating titrants like potassium permanganate (KMnO₄).
Example: Fe²⁺ titrated with KMnO₄
3. Complexometric Titration
Used to determine metal ion concentrations, typically using EDTA (ethylenediaminetetraacetic acid) as the titrant.
Example: Determination of Ca²⁺ and Mg²⁺ in water
4. Precipitation Titration
Relies on the formation of a precipitate during the reaction.
Example: AgNO₃ titrated with Cl⁻ ions (Mohr’s method)

Equipment Needed
- Burette
- Pipette and pipette filler
- Conical (Erlenmeyer) flask
- White tile (to observe color change)
- Stand and clamp
- Funnel
- Beaker with titrant
- Indicator solution
Step-by-Step Titration Procedure
1. Prepare the Apparatus
- Rinse the burette, pipette, and conical flask with the appropriate solutions to avoid dilution errors.
- Fill the burette with the titrant using a funnel, ensuring no air bubbles remain in the nozzle.
2. Measure the Analyte
- Use a pipette to transfer a fixed volume of the analyte into the conical flask.
- Add a few drops of an appropriate indicator to the analyte solution.
3. Start the Titration
- Place the conical flask under the burette on a white tile.
- Slowly release the titrant from the burette while swirling the flask to mix.
4. Watch for the End Point
- Observe a distinct color change indicating the end point.
- Record the volume of titrant used.
5. Repeat for Accuracy
- Repeat the titration several times until you get concordant results (two or more values within 0.1 mL of each other).
- Use the average of the closest values to calculate concentration.
Example Titration Calculation
Given:
- 25.00 mL of HCl is titrated with 0.100 mol/L NaOH
- Average volume of NaOH used: 22.50 mL
Reaction: HCl + NaOH → NaCl + H₂O
Step 1: Calculate moles of NaOH used
0.100 mol/L × 0.02250 L = 0.00225 mol
Step 2: Use molar ratio (1:1)
So, moles of HCl = 0.00225 mol
Step 3: Find concentration of HCl
0.00225 mol ÷ 0.02500 L = 0.0900 mol/L
Tips for Successful Titration
- Swirl continuously to ensure thorough mixing.
- Use a white tile to help observe the color change clearly.
- Go dropwise near the endpoint to avoid overshooting.
- Always record initial and final burette readings accurately.
Applications of Titration
Chemical Analysis in Metallurgy
In metallurgy, automatic chemical titrators play a key role in analyzing metal ores. They help determine elements like iron with high precision. These titrators allow users to control this speed, which reduces errors during analysis. As a result, they improve the reliability and accuracy of metal content assessments.
Education and Student Performance
In classrooms and labs, titration tools like the Titration Colorcam enhance learning. These devices assist students with acid-base experiments by showing the endpoint clearly. This visual aid boosts student confidence and improves scientific performance. Furthermore, it helps educators demonstrate complex reactions more effectively.
Measurement of Ions
Mass titration is a reliable method for measuring ion concentrations in solutions. It works well for substances like Hg(NO₃)₂ and EDTA. Researchers value this technique for its precision, with less than 0.11% relative deviation. Therefore, it serves as a strong alternative to volumetric and gravimetric methods.
Surface Functional Group Analysis
Scientists use titration to analyze surface functional groups on materials like carbon. They neutralize specific groups to assess the degree of functionalization. This technique provides critical insights into material structure and performance. Moreover, it supports advancements in surface chemistry and material design.
Automated and Electrochemical Titration
Modern titration systems now include automation for greater accuracy. Automated titrators identify reaction endpoints and perform calculations independently. These systems reduce human error and increase lab efficiency. In addition, they save time during repetitive or high-volume analysis tasks.
Food and Beverage Industry
Titration is essential in testing food and beverage quality. Analysts use it to measure total acidity and alkalinity. New methods like Flash Titration™ use silicon chips and electrochemical sensors. These innovations eliminate liquid reagents and deliver fast, accurate results for quality control.
Water Quality Testing
Titration plays a vital role in monitoring water quality. Technicians use it to measure pH, acidity, and alkalinity levels. These readings help ensure that water remains safe for consumption and use. Consistent this methods support regulatory compliance and public health.
Industrial Applications
In industries, titration supports both quality control and research. Manufacturers use it to test product composition, such as acidity in edible oils. These titrators deliver repeatable, accurate results for R&D and daily operations. Consequently, they help maintain product standards and improve efficiency.
Conclusion
Titration is a powerful and precise technique used in analytical chemistry. Whether you’re neutralizing an acid or determining metal concentrations in water, mastering the titration process equips you with essential skills for lab accuracy and chemical understanding. By knowing the types, steps, and calculations involved, you can confidently perform titrations and interpret your results.
FAQs
To determine the unknown concentration of a substance in solution.
It depends on the type of titration. Phenolphthalein for strong acid–strong base, methyl orange for strong acid–weak base, etc.
The equivalence point is the actual theoretical point of neutralization; the end point is the observed color change near that point.
At least 3 trials, with at least two results within 0.1 mL of each other for reliable data.
Yes, automatic titrators are used in industry for precision and efficiency.
To get detailed scientific explanations of titration, try Patsnap Eureka.


