
Picric acid, or 2,4,6-trinitrophenol (TNP), is a highly nitrated organic compound known for its explosive properties, yellow crystalline appearance, and historical use in dyes, explosives, and medical applications. Due to its extreme sensitivity to shock, heat, and friction, picric acid requires strict handling and storage protocols to prevent hazardous incidents. This article explores its chemical properties, uses, hazards, and safety considerations.
What is Picric Acid?
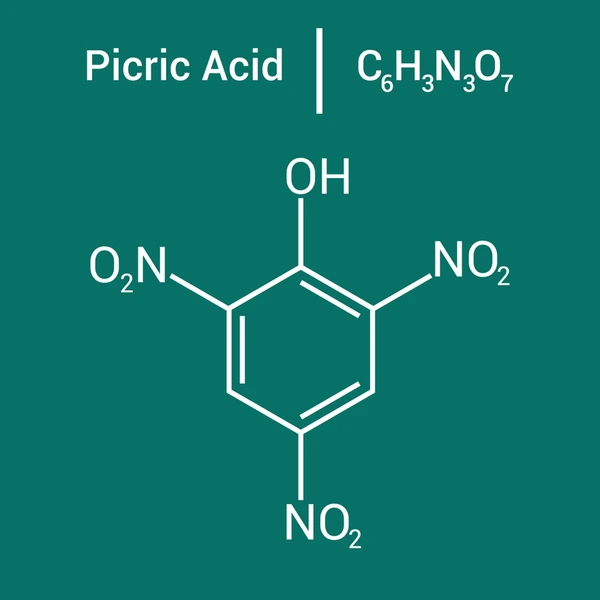
Picric acid is a high-energy, nitrated phenol that was historically used as one of the first high explosives before being replaced by more stable alternatives. It is similar in structure to TNT (trinitrotoluene) but contains a hydroxyl (-OH) group, making it more acidic and water-soluble.

Chemical Properties
- Molecular Formula: C₆H₃N₃O₇
- Molar Mass: 229.10 g/mol
- Appearance: Yellow crystalline solid
- Odor: Odorless, bitter taste
- Density: 1.763 g/cm³
- Melting Point: 122.5°C
- Boiling Point: Decomposes upon heating
- Solubility: Slightly soluble in water, highly soluble in organic solvents
- Acidity (pKa): 0.38 (highly acidic for a phenol)
Physical States
2,4,6-trinitrophenol (TNP) is typically stored as a moist solid or aqueous solution to prevent accidental detonation. When dry, it becomes highly shock-sensitive, making proper moisture control crucial for safe handling.
Uses of Picric Acid
1. Explosives Manufacturing
- Historically used in military explosives under names such as Lyddite and Melinite.
- One of the earliest high explosives used before TNT became the preferred choice.
- Due to instability and sensitivity, its use in modern explosives has significantly declined.
2. Dye and Staining Agent
- Used in the textile and leather industries as a yellow dye.
- Employed in histology and microscopy as a biological tissue stain (e.g., in Bouin’s solution).
3. Medical Applications
- Historically used as an antiseptic and burn treatment during World War I.
- Was included in first-aid kits for treating trench foot and burns, but its medical use has been discontinued due to toxicity concerns.
💡 Curious about the uses of picric acid? Eureka Technical Q&A provides expert insights into its applications in explosives, dyes, and medical treatments, helping you understand its benefits, handling precautions, and industrial significance.
Application Cases
| Product/Project | Technical Outcomes | Application Scenarios |
|---|---|---|
| TNP Detection Compound Council of Scientific & Industrial Research | Novel compound for colorimetric and fluorescence detection of 2,4,6-trinitrophenol | Detection of explosive materials in security and forensic applications |
| Fluorescent Polytannic Acid Nano-dots Qingdao Agriculture University | High sensitivity picric acid detection with a limit of 0.17 μg/L, excellent chemical and optical stability | Rapid and sensitive detection of picric acid in environmental and security monitoring |
| Bis-pyrene-based Fluorometric Sensor National Institute of Technology Jamshedpur | Fluorometric detection of picric acid using a novel bis-pyrene-based sensory molecule | Trace detection of picric acid in forensic and environmental analysis |
| Picric Acid Derivatives Synthesis University of Engineering & Technology of Lahore | Synthesis and characterization of picric acid derivatives including picramic acid and sodium picramate | Research and development in dye industry and explosive materials |
| Metal Surface Passivation Method SixRing, Inc. | Passivation of metal surfaces using modified Caro’s acid compositions containing peroxides and sulfonic acids | Corrosion protection in metal processing and manufacturing industries |
Hazards and Safety Considerations
1. Explosive Hazards
- Dry picric acid is highly explosive and can detonate upon shock, friction, or heat exposure.
- Forms highly unstable metal picrates when in contact with metals such as copper, iron, and zinc, increasing explosion risks.
- Even small quantities of dried 2,4,6-trinitrophenol (TNP) can detonate spontaneously under mechanical stress.
2. Health Hazards
- Skin and Eye Irritation: Causes severe irritation and can stain skin yellow upon contact.
- Respiratory Risks: Inhalation of dust or vapors can lead to coughing, shortness of breath, and lung irritation.
- Toxicity: Chronic exposure may cause liver and kidney damage and affect the nervous system.
3. Environmental Impact
- Can contaminate soil and water sources, leading to environmental hazards.
- Releases toxic nitrogen oxides (NOx) upon combustion.
Safe Handling and Storage Practices
1. Personal Protective Equipment (PPE)
- Eye Protection: Safety goggles or face shields to prevent eye contact.
- Skin Protection: Acid-resistant gloves and protective clothing to prevent skin exposure.
- Respiratory Protection: Proper ventilation or respirator use when handling in confined spaces.
2. Engineering Controls
- Ventilation: Always handle in a fume hood or well-ventilated area to minimize inhalation risks.
- Spill Containment: Ensure proper containment measures to prevent dust formation and spread.
3. Storage Guidelines
- Moist Storage: Must be stored in a moist state (at least 10% water content) to prevent drying.
- Non-Metallic Containers: Avoid metal containers to prevent the formation of shock-sensitive metal picrates.
- Cool, Dry Environment: Keep in a temperature-controlled and well-ventilated storage area.
4. Emergency Procedures
- Spill Cleanup: Always keep the material wet when handling spills to prevent accidental detonation.
- Fire Response: Use water spray or dry chemical powder—never use organic fire suppressants.
- First Aid: In case of skin or eye contact, rinse immediately with water and seek medical attention.
FAQs About Picric Acid
1. Why is picric acid dangerous?
Picric Acid is highly explosive when dry and can form unstable metal picrates when in contact with metals. This makes it one of the most sensitive explosive compounds used historically.
2. Can picric acid be safely used today?
Yes, but only under strict safety measures. It must be stored in moist conditions, handled with non-metallic tools, and kept in a controlled environment.
3. What happens if picric acid dries out?
Dried 2,4,6-trinitrophenol (TNP) becomes extremely shock-sensitive, meaning even a small vibration or impact can cause spontaneous detonation.
4. How should picric acid be disposed of?
It must be neutralized with an alkaline solution and disposed of as hazardous waste following regulatory guidelines. Improper disposal can lead to serious environmental hazards.
5. What precautions should be taken when handling picric acid?
Always wear PPE, store it moist, avoid metal contact, and handle in a well-ventilated area. Regularly inspect containers to ensure the substance has not dried out or crystallized.
Conclusion
Picric acid has been widely used in explosives, dyes, and medical applications, but its high sensitivity and explosive nature require extreme caution in handling, storage, and disposal. While it is no longer commonly used in modern applications, it remains a critical chemical in specialized industries. Adhering to proper safety protocols ensures that 2,4,6-trinitrophenol (TNP) can be used effectively while minimizing risks.
To get detailed scientific explanations of Picric Acid, try Patsnap Eureka.


