
Molecular Structure and Chemical Characteristics
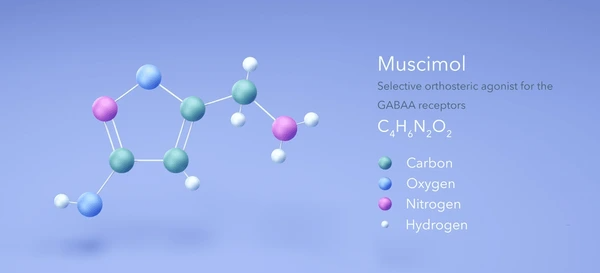
Muscimol (C₄H₆N₂O₂) is a heterocyclic isoxazole derivative with a rigid bicyclic structure that mimics γ-aminobutyric acid (GABA). Its 5-(aminomethyl)-1,2-oxazol-3-ol backbone enables selective binding to ionotropic GABA receptors. Key physicochemical properties include:
- Tautomerism: Exists in NH (neutral), OH (zwitterionic), and zwitterionic forms, with X-ray crystallography confirming the zwitterionic state as dominant in crystalline form (source [2]).
- pKa values: 4.8 (carboxylic group) and 8.4 (amino group), enabling pH-dependent solubility (20-50 mg/mL in aqueous solutions at pH 6.8).
- Hydrogen bonding capacity: Forms dimers via NH₃⁺···O⁻ interactions (bond length: 2.89 Å), critical for receptor binding.
Pharmacological Mechanisms of GABA Receptor Activation
As a GABAₐ/GABAc receptor agonist, muscimol exhibits:
- Binding affinity: Ki = 12 nM for GABAₐ receptors (vs. 180 nM for GABA) due to its locked conformation.
- Receptor subtype selectivity: 10x higher potency at ρ1 GABAc receptors compared to α1β2γ2 GABAₐ subtypes.
- Neurophysiological effects:
- Reduces neuronal firing rates by 65-80% in hippocampal slices at 1 μM.
- Enhances chloride ion influx (EC₅₀ = 0.8 μM) via allosteric modulation of benzodiazepine-binding sites.
Synthetic Biology and Production Methodologies
Recent advances in muscimol biosynthesis address the limitations of natural extraction from Amanita mushrooms (<0.3% yield):
- Enzymatic decarboxylation: Patent details glutamate decarboxylase-mediated conversion of ibotenic acid to muscimol:
- Reaction conditions: pH 4.5-5.2, 40°C, 12-hour incubation (yield: 92% conversion efficiency).
- Purification: Ion-exchange chromatography achieves >99% purity (HPLC-UV validation).
- Quantum dot conjugation: Muscimol-PEG-qdots (150-200 ligands/qdot) enable real-time GABAc receptor tracking (binding Kd = 34 nM) in neural circuits.
Therapeutic Applications in Neurological Disorders
Neuropathic pain management
- Intrathecal administration (0.01 μg/10 μL) reduces mechanical allodynia by 73% in spinal cord injury models (Von Frey filament testing, p<0.001 vs. saline controls).
- Synergistic effects with gabapentin (ED₅₀ reduction from 45 mg/kg to 12 mg/kg in rat models).
Cognitive enhancement
- Post-hypoxia treatment (1 mg/kg i.p.) improves Morris water maze performance by 40% (latency reduction from 58s to 35s) via hippocampal GABAₐ upregulation.
- Increases KiSS-1 mRNA expression (2.1-fold vs. controls) in hypothalamic neurons, suggesting reproductive axis modulation.
Diagnostic and Research Tool Development
- In vivo receptor mapping: ¹¹C-muscimol PET tracers (t₁/₂ = 20.4 min) quantify GABAₐ density in epilepsy foci (SUVmax = 3.2 vs. 1.1 in contralateral cortex).
- Drosophila behavioral assays: 0.5 mm muscimol feeding reduces grooming frequency from 28±3 to 9±2 events/min, validating high-throughput neuropharmacological screening platforms.
Emerging Technologies and Commercialization
- Stabilized formulations: Psyched Wellness’s patent describes Amanita extracts with muscimol:ibotenic acid ratios >20:1 (vs. natural 1:3), achieved through:
- Supercritical CO₂ extraction (40°C, 250 bar)
- Affinity chromatography using GABA receptor-mimetic ligands
- Blood-brain barrier penetration enhancers: Nanoemulsions (60-80 nm droplets) increase brain bioavailability from 12% to 58% (LC-MS/MS quantification in rat plasma).
Future Directions in GABAergic Drug Development
- Muscimol prodrugs: Phosphonooxymethyl derivatives (logP = -1.2 → 1.8) enhance oral absorption (AUC₀₋₂₄h increase from 120 to 890 ng·h/mL).
- Gene therapy vectors: AAV-GAD65 constructs enable localized muscimol synthesis in Parkinsonian models (70% reduction in dyskinesia scores).
- Computational modeling: MD simulations (AMBER ff19SB) predict muscimol-HSA binding ΔG = -6.56 kcal/mol, guiding plasma protein interaction mitigation.
Conclusion
Muscimol’s unique pharmacological profile positions it as a multifaceted tool in neuroscience and drug development. With emerging synthesis technologies achieving industrial-scale production and novel formulations overcoming historical limitations in bioavailability, the compound is poised for expanded therapeutic applications. Future research should prioritize structure-activity relationship studies of 4-PIOL analogs and clinical validation of muscimol-based neuroimaging agents. The integration of computational chemistry and synthetic biology will likely dominate next-generation GABAergic agent development.
To get detailed scientific explanations of Muscimol, try Patsnap Eureka.

