
Catalytic hydrogenation is a chemical reaction that adds hydrogen (H₂) to unsaturated compounds using a catalyst, typically transforming double or triple bonds into single bonds. This article explains what catalytic hydrogenation is, how it works, what catalysts are used, and why it plays a vital role in industries from food processing to pharmaceuticals.
Definition of Catalytic Hydrogenation
Whether you’re learning about catalytic hydrogenation mechanisms or exploring its industrial applications, Eureka Technical Q&A connects you with chemical experts who can simplify catalysts, reaction conditions, and selectivity—helping you fully understand this essential organic chemistry process.
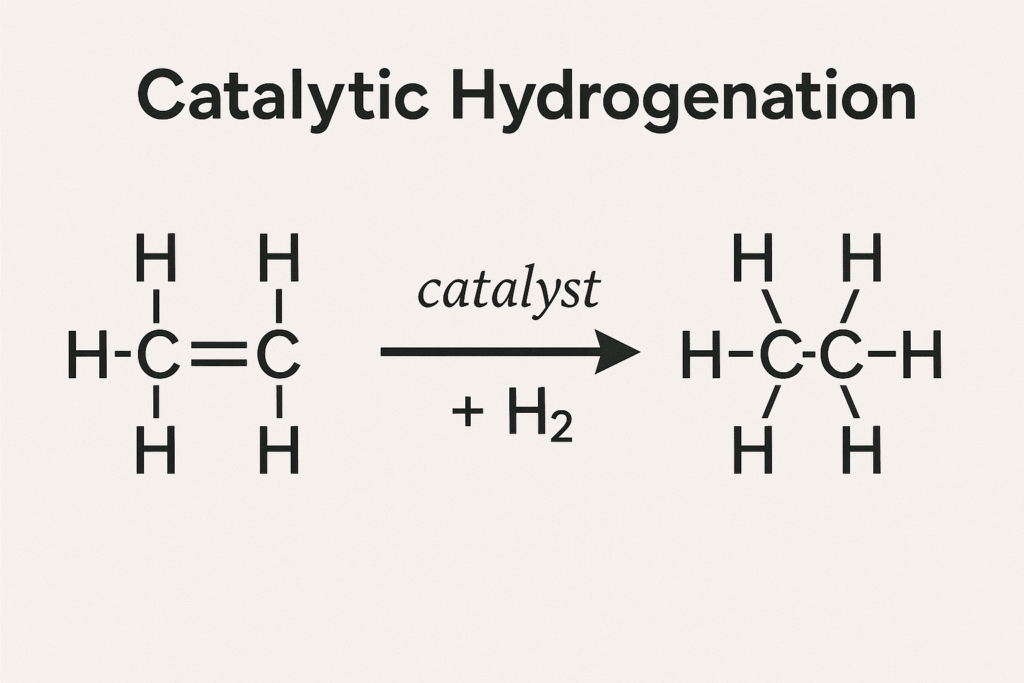
In catalytic hydrogenation, the catalyst facilitates the addition of hydrogen gas (H₂) across multiple bonds—usually carbon–carbon double (C=C) or triple (C≡C) bonds. The purpose is to reduce unsaturated compounds into saturated ones, often increasing chemical stability and altering physical properties.
Basic Reaction Format
General form of catalytic hydrogenation:
RCH=CH₂ + H₂ → RCH₂CH₃ (in presence of catalyst)
- The reaction involves breaking the π bonds and adding hydrogen atoms to each carbon.
- Common catalysts include palladium (Pd), platinum (Pt), and nickel (Ni).
Key Components of Catalytic Hydrogenation
1. Substrate
- Typically alkenes (C=C) or alkynes (C≡C)
- Can also include ketones, aldehydes, or nitriles in some contexts
2. Hydrogen Gas (H₂)
- Acts as the reducing agent
- Must be activated by the catalyst for the reaction to proceed
3. Catalyst
- Commonly used:
- Palladium on carbon (Pd/C)
- Platinum (Pt)
- Raney nickel (Ni)
- Surface of the catalyst facilitates the adsorption and activation of hydrogen
Mechanism of Catalytic Hydrogenation
- Adsorption of Hydrogen
- H₂ molecules bind to the catalyst surface and dissociate into atomic hydrogen.
- Adsorption of the Unsaturated Substrate
- The alkene or alkyne also adsorbs onto the catalyst surface.
- Addition of Hydrogen Atoms
- Hydrogen atoms are transferred to the carbon atoms of the unsaturated bond.
- Desorption
- The now saturated compound detaches from the catalyst surface.
This mechanism occurs on the catalyst surface, and the reaction proceeds under mild to moderate temperatures and pressures.
Types of Catalytic Hydrogenation
1. Homogeneous Catalytic Hydrogenation
- Uses soluble catalysts (e.g., Wilkinson’s catalyst: RhCl(PPh₃)₃)
- Allows fine-tuned selectivity, useful in pharmaceuticals
2. Heterogeneous Catalytic Hydrogenation
- Uses solid catalysts (e.g., Pd/C, PtO₂)
- Easy to separate products and scale up for industrial processes
Industrial and Real-World Applications
1. Food Industry
- Hydrogenation of vegetable oils to create margarine and shortenings
- Converts liquid oils (unsaturated) into semi-solids (saturated fats)
2. Petrochemical Industry
- Hydrotreating and hydrocracking of fuels to remove sulfur and reduce unsaturation
3. Pharmaceuticals
- Used in the synthesis of active pharmaceutical ingredients (APIs)
- Enables selective reduction of double/triple bonds in complex molecules
4. Fine Chemicals
- Useful in dye, perfume, and specialty chemical production
- Allows precise modifications of molecular structures
Cis and Trans Hydrogenation
- Syn addition (both hydrogens added to same side) typically gives cis-products
- Some catalysts can be modified to favor cis or trans products selectively
Example:
Lindlar’s catalyst (Pd with lead acetate and quinoline) selectively hydrogenates alkynes to cis-alkenes.
Safety and Environmental Considerations
- Hydrogen gas is flammable and explosive—requires careful handling.
- Catalysts like Pd and Pt are expensive and must be recovered and recycled.
- Industrial hydrogenation often operates under high pressure (10–100 atm), necessitating robust equipment.
Advantages of Catalytic Hydrogenation
- Efficient and rapid method of reduction
- Highly selective under the right conditions
- Works on a wide range of functional groups
- Scalable from lab to industrial level
Limitations and Challenges
- Not all functional groups tolerate hydrogenation conditions
- Over-hydrogenation may occur if not carefully monitored
- Some reactions require high pressure or temperature
- Catalyst poisoning (e.g., by sulfur compounds) can deactivate catalysts
Common Catalysts Used
Metal Catalysts
Metal catalysts include noble metals such as palladium, platinum, and rhodium. These are widely used in automotive catalytic converters to reduce harmful emissions. In addition, nickel-based catalysts play a major role in steam reforming, where they help produce synthesis gas (syngas). Their high activity and durability make them essential in many industrial processes.
Acid Catalysts
Chemists commonly use acid catalysts in reactions like dehydration, hydration, and polymerization. Typical examples include sulfuric acid, phosphoric acid, and solid acid resins. These catalysts are especially valuable in the petrochemical industry, where they drive processes such as alkylation and polymerization with high efficiency.
Base Catalysts
Base catalysts are ideal for reactions needing a basic environment, such as polycondensation and hydrolysis. Alkali metals like sodium and potassium, along with organic amines, are commonly used. These catalysts are popular in synthetic chemistry and material production due to their strong basic properties.
Zeolite Catalysts
Zeolite catalysts are microporous aluminosilicates used in several industrial applications. ZSM-5 and other zeolites offer high surface area and strong acidity. These features make them effective in catalytic pyrolysis and other transformations that benefit from shape-selective catalysis.
Metal Oxide Catalysts
Metal oxide catalysts consist of oxides of copper, iron, nickel, manganese, chromium, and cobalt. Industries widely use these in catalytic oxidation reactions. Environmental and chemical sectors favor them for their stability, reactivity, and long-lasting performance.
Organic-Inorganic Hybrid Catalysts
These hybrid catalysts blend organic and inorganic components to deliver enhanced activity and selectivity. They are frequently used in selective oxidation reactions where precision and high yields are critical. This combination approach allows fine-tuning of catalyst properties for specific reactions.
Photoactivated Catalysts
Photoactivated catalysts respond to light activation and are increasingly used in water treatment and advanced chemical synthesis. Their ability to regenerate and recover makes them cost-effective and sustainable. These catalysts are essential in green chemistry and photocatalytic applications.
| Product/Project | Technical Outcomes | Application Scenarios |
|---|---|---|
| HMF Conversion Process Archer-Daniels-Midland Co. | High conversion rates and reduced VOC in coating compositions using nickel and zirconium catalyst system | Industrial production of HMF derivatives with improved environmental safety |
| Diol Derivative Production Nippon Shokubai Co., Ltd. | Cost-effective production of high-purity diol derivatives by eliminating impurities | Industrial-scale production of diol derivatives for various applications |
| Meso-scale Channeled Structured Catalyst Indian Institutes of Technology | High methane conversion and increased throughput in steam reforming processes | Energy-efficient syn gas production in existing steam reforming facilities |
| Radiation-Sensitive Resin JSR Corp. | Enhanced transparency, etching resistance, and process margins in microprocessing | High-resolution and low-stress pattern formation in semiconductor manufacturing |
| Noble Metal Hybrid Catalyst Bayer AG | High selectivity and long operational lifetimes in hydrocarbon oxidation | Efficient industrial processes for selective oxidation of hydrocarbons |
Summary of Catalytic Hydrogenation
| Feature | Description |
|---|---|
| Reaction Type | Reductive addition of hydrogen |
| Common Targets | Alkenes, alkynes, ketones, aldehydes |
| Common Catalysts | Pd/C, Pt, Raney Ni, Rh-based catalysts |
| Reaction Conditions | Often requires pressure and heat |
| Applications | Food, fuels, pharma, fine chemicals |
Conclusion
Catalytic hydrogenation is a cornerstone reaction in both organic chemistry and industrial synthesis. By understanding the role of catalysts, the reaction mechanism, and how to control selectivity, chemists can efficiently reduce compounds for applications ranging from edible fats to life-saving drugs. Its versatility and efficiency make it one of the most powerful tools in the modern chemical toolkit.
FAQs
No. Hydrogenation adds hydrogen to unsaturated bonds, while hydrogenolysis breaks bonds (e.g., C–O or C–C) using hydrogen.
Catalysts activate hydrogen gas and lower the energy barrier for the reaction to proceed efficiently.
Yes, but this usually requires harsh conditions and special catalysts.
It selectively hydrogenates alkynes to cis-alkenes without further reduction to alkanes.
Yes, but they must be recovered and regenerated, especially in industrial setups.
To get detailed scientific explanations of catalytic hydrogenation, try Patsnap Eureka.


