Glycerol, also known as glycerin or 1,2,3-propanetriol, is a simple polyol compound that plays a critical role in both traditional and modern industrial chemistry. Recognized for its hygroscopic nature, non-toxicity, and remarkable chemical stability, glycerol serves as a foundational ingredient across sectors ranging from pharmaceuticals and cosmetics to bioplastics and renewable fuels. With increasing pressure to decarbonize chemical processes and replace fossil-derived feedstocks, glycerol—especially bio-based glycerol—is gaining momentum as a sustainable alternative. This article explores the multifaceted roles of glycerol, comparing its properties with alternatives, surveying current industrial applications, and outlining the latest technological frontiers shaping its future through PatSnap Eureka AI Agent.
What Is Glycerol and Why It Matters
Glycerol (also known as glycerin or propane-1,2,3-triol) is a simple polyol compound with extensive utility across multiple industries. Its molecular formula, C3H8O3, allows it to function as a backbone in lipids and a platform chemical in manufacturing. Naturally derived from fats and oils through saponification or transesterification (e.g., biodiesel production), glycerol offers high biocompatibility, strong hydrogen bonding, and low toxicity—making it ideal for applications ranging from pharmaceuticals and food additives to polymer processing and green solvents.
Due to its renewability and growing availability as a byproduct of biodiesel, glycerol is gaining increasing attention in circular economy strategies, bio-refining, and materials innovation.

Material Composition and Grade Data
Glycerol is categorized into various grades based on purity and intended application:
- USP/FCC Grade Glycerol: >99.7% purity; used in food, cosmetics, and pharmaceuticals.
- Supplier Example: Dow Chemical’s Superol Glycerin
- Technical Grade Glycerol: 95–99% purity; used in antifreeze, resins, and feed.
- Example Product: Emery Oleochemicals Technical Glycerine
- Crude Glycerol: ~40–88% purity; often derived from biodiesel production and contains impurities like methanol, soaps, and salts.
Datasheets typically specify water content, ash content, color (APHA), and fatty acid content. Suppliers such as IOI Oleochemicals, Procter & Gamble Chemicals, and ADM offer detailed material specifications tailored to end-use requirements.
Key Properties That Define Glycerol
- Molecular Weight: 92.09 g/mol
- Viscosity: ~1410 cP at 20°C
- Boiling Point: ~290°C (decomposes)
- Melting Point: 17.8°C
- Hygroscopicity: Strong moisture retention
- Solubility: Completely miscible with water and alcohols; insoluble in hydrocarbons
- Thermal Stability: Decomposes before boiling; prone to oxidation above 180°C
Its triol structure enables glycerol to serve as a humectant, plasticizer, and hydrogen donor in chemical reactions. Moreover, its hydroxyl groups enable esterification, etherification, and acetal formation—expanding its utility in formulation chemistry.
Core Applications Across Industries
Pharmaceutical & Healthcare
Glycerol functions as a solvent, excipient, and preservative in cough syrups, lozenges, and oral care products. Due to its osmotic and lubricating properties, it is used in suppositories, laxatives, and skin creams. Long-tail keyword examples: glycerol in pharmaceutical formulations, USP glycerin in oral solutions, glycerol-based dermatological emulsions.
Food & Beverage
As a GRAS (Generally Recognized as Safe) additive, glycerol acts as a sweetener, humectant, and stabilizer in processed foods, baked goods, and beverages. It also serves as a cryoprotectant in frozen foods. Use case: Moisture retention in gluten-free baked formulations.
Polymer & Plastics
Glycerol acts as a bio-based plasticizer in thermoplastic starch, PVA, and PLA blends. It enhances flexibility and processability in biodegradable films and foams. It is also a precursor in the synthesis of epichlorohydrin, a key epoxy resin monomer.
Personal Care & Cosmetics
Used extensively in lotions, shampoos, toothpaste, and soaps for its hydrating and viscosity-modifying properties. Its compatibility with surfactants and emulsifiers makes it indispensable in stable cosmetic formulations.
Industrial Chemicals & Lubricants
Glycerol is an intermediate in producing propylene glycol, acrolein, and other industrial chemicals. In lubrication, it functions in water-based metalworking fluids and as a component of anti-freeze systems due to its non-toxicity.
Energy & Biofuels
Crude glycerol is increasingly used as a carbon source for anaerobic digestion, biogas production, and glycerol steam reforming for hydrogen generation. Benefit: Adds value to biodiesel waste stream and supports renewable energy loops.
Comparative Advantages and Limitations
Glycerol’s broad utility stems from its unique combination of physical and chemical properties, but like any industrial compound, it presents both strengths and challenges.
Advantages:
- Biocompatibility and Non-Toxicity: Glycerol is generally recognized as safe (GRAS) by regulatory agencies, making it suitable for direct human contact in food, cosmetics, and pharmaceuticals.
- Hydrophilicity and Moisture Retention: Its strong affinity for water enables its use as a humectant in personal care and food formulations.
- Chemical Versatility: With three hydroxyl groups, glycerol is an ideal platform for derivatization into epichlorohydrin, propylene glycol, and polyglycerols.
- Renewable Sourcing: It is abundantly produced as a by-product in biodiesel manufacturing, offering a carbon-neutral alternative to petroleum-derived polyols.
- Thermal and Oxidative Stability: Glycerol can be stored and processed under ambient conditions without rapid degradation.
- Lubricity and Plasticizing Effect: In polymer systems, glycerol improves flexibility, softness, and flow characteristics.
Limitations:
- High Hygroscopicity: While beneficial in moisturizing applications, excessive water absorption can cause product instability or viscosity drift in formulations.
- Low Volatility and High Viscosity: Glycerol’s thick, syrupy texture can be a limitation in processes requiring volatility or rapid diffusion.
- Combustion Inefficiency: As a fuel precursor, its calorific value is lower than hydrocarbon fuels, reducing its standalone utility in energy applications.
- Microbial Susceptibility: Its water-retentive nature makes glycerol-containing formulations prone to microbial contamination unless preserved.
- Overproduction in Biodiesel Industry: The surplus glycerol supply can depress prices and discourage investment in high-value derivative development.
Comparison with Similar Compounds:
| Property | Glycerol | Propylene Glycol | Ethylene Glycol | Sorbitol |
|---|---|---|---|---|
| Toxicity | Non-toxic | Low, but not GRAS | Toxic (oral) | Non-toxic |
| Renewable Availability | High (from biodiesel) | Moderate (bio-PG emerging) | Low | Moderate |
| Viscosity | High | Moderate | Low | Very high |
| Applications | Broad | Solvent, antifreeze | Antifreeze, plastics | Food, pharma |
| Biodegradability | Excellent | Good | Poor | Good |
Glycerol stands out in safe-use applications and as a platform for sustainable innovation, though technical and economic optimization is needed to expand its high-performance applications.
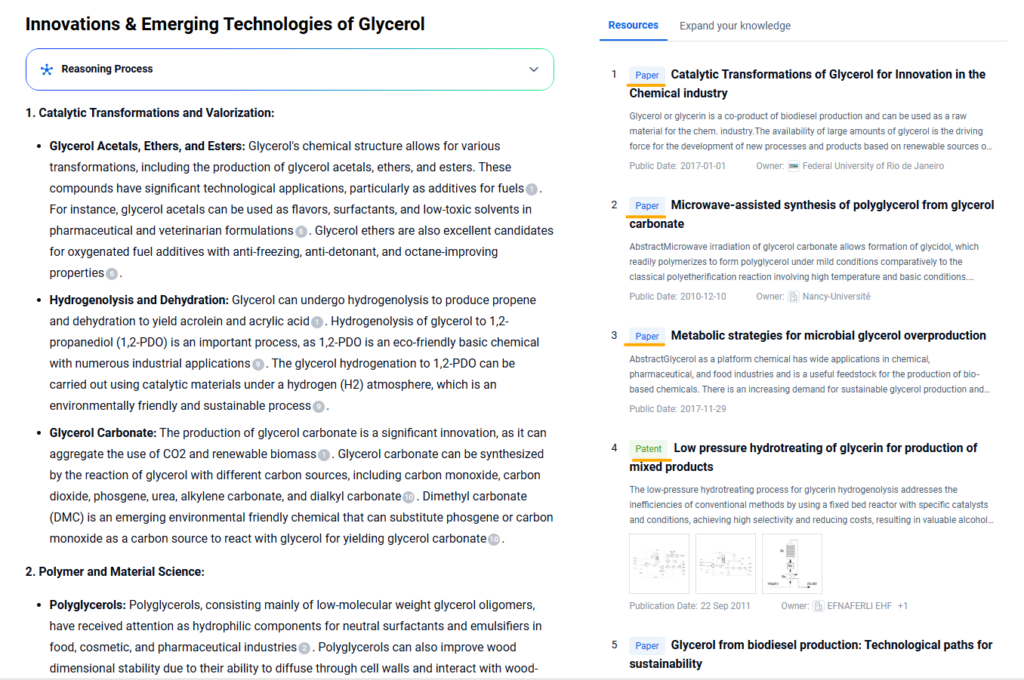
Innovations & Emerging Technologies of Glycerol
As glycerol transitions from a biodiesel by-product to a value-added chemical feedstock, multiple innovations are reshaping its industrial relevance:
Catalytic Upgrading to Epichlorohydrin and Glycidol
Recent advances in heterogeneous catalysis have enabled more efficient conversion of glycerol into epichlorohydrin and glycidol, critical intermediates in epoxy resins and bio-based surfactants. These technologies reduce reliance on petroleum-derived propylene.
Microbial Fermentation into Biopolymers
Engineered microbial strains are being developed to convert crude glycerol into polyhydroxyalkanoates (PHAs) and succinic acid, offering biodegradable plastic alternatives. This positions glycerol as a key enabler of circular bioeconomy platforms.
Electrocatalytic Hydrogen Production
Glycerol-assisted water electrolysis is being explored to lower the energy demand of hydrogen generation. The glycerol acts as a sacrificial anode component, increasing efficiency while producing valuable co-products like formic acid or dihydroxyacetone.
Glycerol Carbonate Synthesis
Novel routes involving transesterification of glycerol with dimethyl carbonate produce glycerol carbonate, a promising green solvent and intermediate for non-isocyanate polyurethanes (NIPUs).
Smart Hydrogel Materials
Glycerol-infused polymer networks are showing potential in wearable electronics, biosensing, and controlled drug delivery due to their self-healing and moisture-responsive behavior.
3D Printing and Additive Manufacturing
In bioinks and hydrogels, glycerol enhances printability and cell viability, especially in extrusion-based 3D bioprinting platforms targeting regenerative medicine.
CO₂ Valorization
Emerging research explores coupling glycerol oxidation with CO₂ fixation reactions to yield cyclic carbonates or oxalates under mild conditions, contributing to carbon-neutral chemical synthesis pathways.
These developments reflect glycerol’s potential as both a feedstock and functional component in next-generation technologies.
Industry Challenges and Future Outlook
Key industry challenges include managing oversupply from biodiesel production, purifying crude glycerol cost-effectively, and scaling up catalytic conversion pathways. However, global demand for sustainable solvents, biobased polyols, and safe food/pharma additives continues to grow.
Emerging glycerol valorization strategies—such as glycerol-to-acrylic acid conversion and anaerobic glycerol fermentation—could transform glycerol from a byproduct into a cornerstone of green chemistry.
Conclusion
Glycerol’s versatility, renewability, and low toxicity make it a cornerstone of bio-based materials innovation. Whether used in cosmetics, pharmaceuticals, biodegradable plastics, or energy systems, it bridges performance with sustainability.
FAQs
A1: They refer to the same chemical compound. “Glycerin” is the commercial name, often used for refined or cosmetic/pharma-grade materials.
A2: Yes, USP and FCC grades of glycerol are approved for food and pharmaceutical use.
A3: Yes, it’s used in hydrogel-based bioinks as a plasticizer and viscosity enhancer in bioprinting applications.
A4: Common techniques include distillation, ion exchange, acidification, and membrane filtration to remove methanol, salts, and organics.
Want to uncover patent trends, supplier landscapes, or R&D breakthroughs in biobased polyols like glycerol?
👉 Explore deeper insights using PatSnap Eureka AI Agent—your AI-powered platform for materials intelligence, competitive landscaping, and innovation scouting.