
Understanding the differences between entropy and enthalpy is crucial in thermodynamics, as both play essential roles in energy transfer and chemical processes. Entropy refers to the measure of disorder or randomness in a system, while enthalpy relates to the total heat content or energy in a system. Though closely linked, these concepts describe distinct properties and have unique applications in science and engineering. This article explores the key differences between entropy vs. enthalpy, their definitions, and how they influence various processes, helping you grasp these fundamental thermodynamic principles.
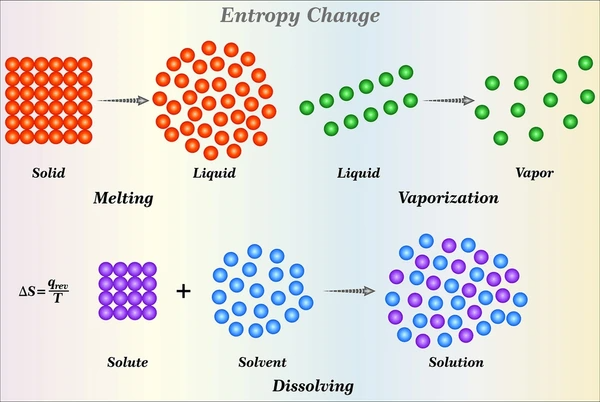
What Is Entropy?
Entropy measures the disorder, randomness, or uncertainty within a system. Rudolf Clausius first introduced the concept in thermodynamics to describe the direction of natural processes. It also quantifies the energy in a system that cannot perform work.
In statistical mechanics, entropy links to the number of microscopic configurations corresponding to a system’s macroscopic state. Ludwig Boltzmann defined entropy using the logarithm of these microstates, connecting it to the fundamental behavior of particles.
Entropy plays a crucial role in understanding energy transfer, natural processes, and the behavior of systems at both macroscopic and microscopic levels.
What Is Enthalpy?
Enthalpy represents the total energy content of a system and is a key concept in thermodynamics. It combines the system’s internal energy with the energy related to its pressure and volume. Mathematically, enthalpy (H) is defined as:
H = U + PV
Where:
- H = Enthalpy
- U = Internal energy
- P = Pressure
- V = Volume
Enthalpy is particularly useful in chemical thermodynamics for simplifying energy calculations in processes at constant pressure. Many chemical reactions and natural processes occur under these conditions, making enthalpy a practical tool for analyzing energy changes. Whether dealing with reactions in a lab or large-scale industrial processes, enthalpy helps describe energy transformations effectively.
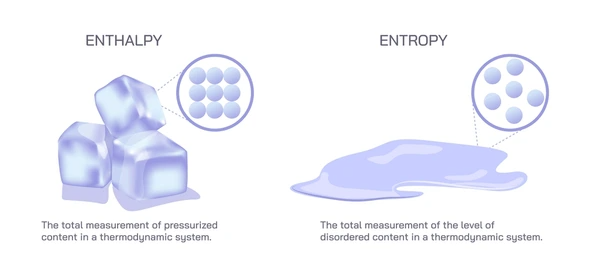
Comparing Entropy and Enthalpy: Key Distinctions
Nature
Entropy measures the system’s internal disorder or randomness. It reflects how disorganized the particles or energy within the system are. On the other hand, enthalpy represents the heat content of a system and is a form of energy associated with heat transfer.
Units
Entropy is measured in joules per kelvin (J/K) or kg·m²·s⁻²·K⁻¹ in SI units. Enthalpy, in contrast, uses joules per mole (J/mol) as its standard unit.
Behavior
Entropy naturally increases in spontaneous processes, illustrating the universal tendency toward greater disorder. Enthalpy does not have a specific direction of change. Instead, it varies depending on the type of process, such as whether heat is absorbed or released.
Interrelation
Entropy and enthalpy work together in thermodynamics through the Gibbs free energy equation: G = H – TS. Here, G determines a process’s spontaneity, with enthalpy (H) and entropy (S) balancing against temperature (T) to influence the outcome.
Real-World Applications of Entropy and Enthalpy
Entropy in Real-World Applications
- Heat Transfer Systems: Entropy is essential for designing efficient heat transfer systems by helping to minimize thermal energy losses. It plays a critical role in improving heating and cooling system efficiency. In industrial applications, engineers use entropy analysis to create better heat exchangers and insulation materials, reducing energy waste and improving performance.
Enthalpy in Real-World Applications
- Power Generation: Enthalpy is crucial in power generation, particularly in steam turbines. The enthalpy change of steam during expansion determines turbine efficiency. By optimizing enthalpy, engineers can increase energy output while reducing fuel consumption, making power plants more cost-effective and environmentally friendly.
- Chemical Reactions: In chemical engineering, enthalpy helps analyze energy changes during reactions, such as refining, petrochemical processes, and pharmaceutical production. By understanding enthalpy changes, engineers can optimize reactions, leading to more efficient and cost-effective industrial processes.
- Material Science: Enthalpy is vital in studying phase transitions and material properties. For example, in metallurgy, it helps analyze energy changes during the heating and cooling of metals. This understanding is crucial for processes like casting, forging, and creating high-performance materials.
To get detailed scientific explanations of Entropy vs. Enthalpy, try Patsnap Eureka.

