
Overview
Entresto (Sacubitril/Valsartan) represents a significant advancement in the heart failure treatment market in the USA. The total number of approved drugs in this therapeutic area is 1, highlighting Entresto’s unique position as a first-in-class angiotensin receptor-neprilysin inhibitor (ARNI). Developed by Novartis, Entresto has demonstrated substantial clinical benefits in reducing cardiovascular death and heart failure hospitalizations. The drug maintains strong patent protection through 2037 for certain aspects, with initial approvals dating back to 2015. With its novel mechanism combining AT1R antagonism and enkephalinase inhibition, Entresto addresses a critical unmet need in the management of heart failure with reduced ejection fraction.
Detailed Description
Drug Information
Entresto (Sacubitril/Valsartan) was developed by Novartis Pharma AG and is approved in the USA for the treatment of chronic heart failure and left ventricular systolic dysfunction.
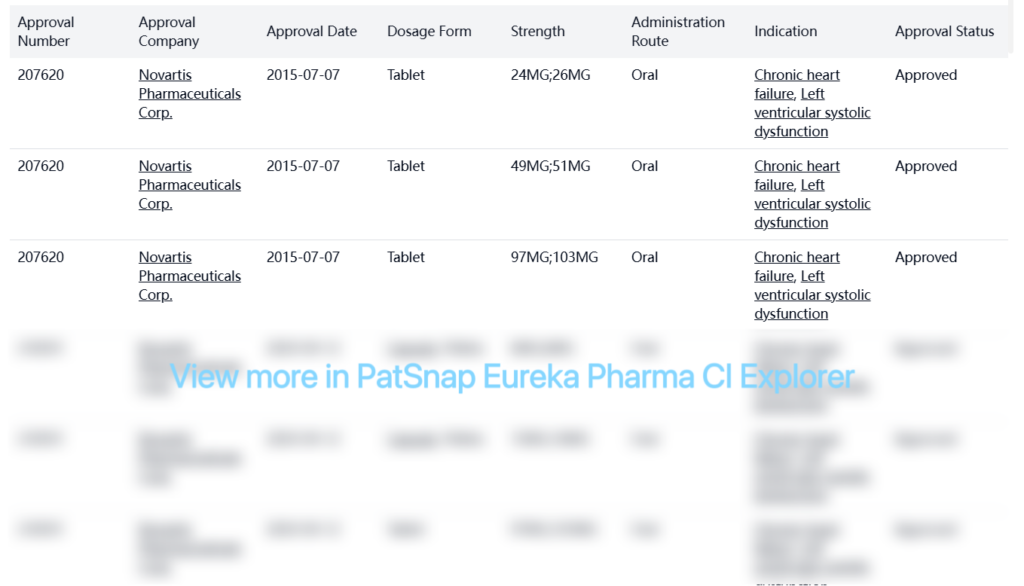
Special Review
| Organization | Indication | Special Review | Country | Approval Date |
|---|---|---|---|---|
| Novartis Pharmaceuticals Corp. | Heart Failure | Fast Track | United States | 2014-06-30 |
Patent Statement Information
| Trade Name | Submission Date | ANDAs Submit Number | 180-day Status | Decision Posting Date | First Applicant Approval Date |
|---|---|---|---|---|---|
| Entresto | 2019-07-08 | 18 | Deferred | 2024-06-10 | 2024-05-28 |
Registration Patent Barrier Analysis
FDA Orange Book Patents
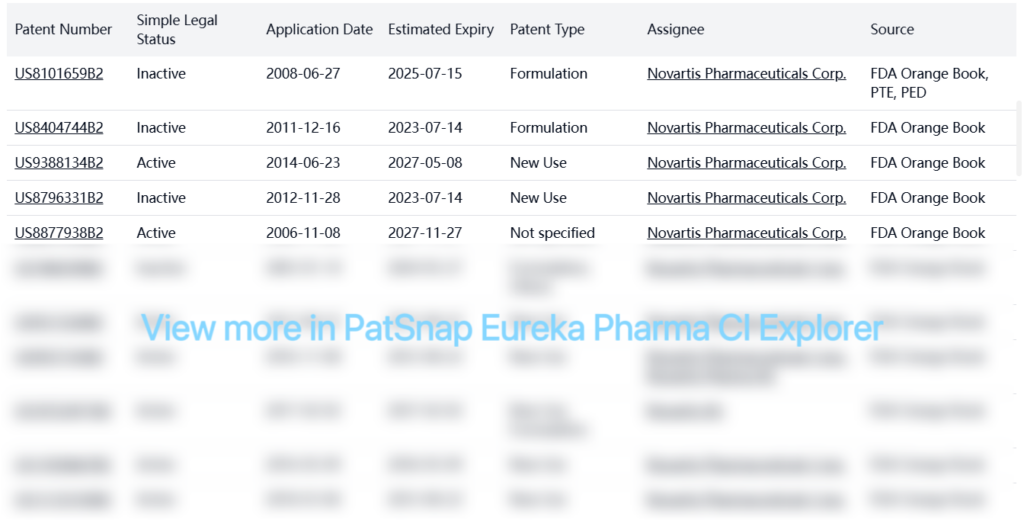
PTE Patents
| Patent Number | Simple Legal Status | Application Date | Estimated Expiry | Patent Type | Assignee | Source |
|---|---|---|---|---|---|---|
| US8101659B2 | Inactive | 2008-06-27 | 2025-07-15 | Formulation | Novartis Pharmaceuticals Corp. | FDA Orange Book, PTE, PED |
| US20080262059A1 | Inactive | 2008-06-27 | 2025-07-15 | New Use, Formulation, Others | Novartis Pharmaceuticals Corp. | PTE, PED |
PED Patents
| Patent Number | Simple Legal Status | Application Date | Estimated Expiry | Patent Type | Assignee | Source |
|---|---|---|---|---|---|---|
| US8101659B2 | Inactive | 2008-06-27 | 2025-07-15 | Formulation | Novartis Pharmaceuticals Corp. | FDA Orange Book, PTE, PED |
| US20080262059A1 | Inactive | 2008-06-27 | 2025-07-15 | New Use, Formulation, Others | Novartis Pharmaceuticals Corp. | PTE, PED |
Other Patent Barrier Analysis
Entresto is protected by numerous patents owned by Novartis and other companies covering various aspects including formulation, process, and new uses. Key non-original patents that may affect generic entry include:
Process Patents
| Patent Number | Simple Legal Status | Application Date | Estimated Expiry | Patent Type | Assignee |
|---|---|---|---|---|---|
| US20180273493A1 | Active | 2016-02-06 | 2036-02-20 | Process | TIANISH LABORATORIES PTE LTD |
| US9242927B2 | Active | 2014-01-24 | 2030-03-23 | Process | Novartis AG, Zhejiang Jiuzhou Pharmaceutical Co., Ltd. |
| US20180318259A1 | Active | 2016-09-01 | 2036-09-01 | New Use, Product Derivative, Process | Chengdu Easton Biopharmaceuticals Co., Ltd. |
Crystal Form Patents
| Patent Number | Simple Legal Status | Application Date | Estimated Expiry | Patent Type | Assignee |
|---|---|---|---|---|---|
| CN105753732A | Active | 2016-03-18 | 2036-03-18 | New Use, Drug Combination, Process, Crystal Form | Nanjing Yixinhe Pharmaceutical Technology Co., Ltd. |
| EP3229799A4 | Inactive | 2015-12-08 | 2035-12-08 | Crystal Form | Suzhou Pengxu Pharmatech Co., Ltd. |
Formulation Patents
| Patent Number | Simple Legal Status | Application Date | Estimated Expiry | Patent Type | Assignee |
|---|---|---|---|---|---|
| US10722471B2 | Active | 2017-02-02 | 2037-02-02 | New Use, Formulation | Novartis AG |
| CN111358783A | Active | 2018-12-25 | 2038-12-25 | Formulation | Beijing Winsunny Pharmaceutical Co., Ltd. |
| CN105748420B | Active | 2016-03-04 | 2036-03-04 | Formulation | Shandong Academy of Biomedical Sciences Co., Ltd. |
Clinical Results
Based on the FDA Label Clinical Insight, the following clinical results were reported for Entresto:
1. Pharmacokinetic and Drug Interaction Experiments
- In Vitro Studies on Transporters
- Evaluated inhibition potential of sacubitril on the OATP1B1 and OATP1B3 transporters [1]
- Dedicated Drug–Drug Interaction Studies
- Clinical pharmacokinetic experiments assessed co-administration with common medications
- Medications like furosemide, digoxin, levonorgestrel, amlodipine, and metformin showed minimal interactions
- Warfarin, carvedilol, ethinyl estradiol, atorvastatin, and sildenafil caused slight to moderate changes in pharmacokinetic profiles.
2. Clinical Trials in Heart Failure
- PARADIGM-HF Study
- Multinational, randomized, double-blind trial enrolling 8,442 adult patients with symptomatic chronic heart failure (NYHA class II–IV) and systolic dysfunction (LVEF ≤40%)
- Sequential single-blind run-in periods with enalapril followed by Sacubitril/Valsartan
- Primary endpoint: time to first occurrence of either cardiovascular death or hospitalization for heart failure
- Patients followed for median duration of 27 months (up to 4.3 years) [2]
- Adverse Reactions Assessment
- During enalapril run-in: 10.5% discontinued (5.6% due to adverse events)
- During Sacubitril/Valsartan run-in: 10.4% discontinued (5.9% due to adverse events) [4]
Infringement Cases
Based on the Generic Drug Infringement News search, no patent infringement incidents involving sacubitril/valsartan were identified.
Policy and Regulatory Risk Warning
After a comprehensive search, Entresto may be subject to regulatory exclusivity periods in the USA. The initial approval in 2015 would typically confer 5 years of new chemical entity exclusivity, which has now expired. However, the new pediatric formulations approved in 2024 may have additional exclusivity periods. Additionally, the orphan drug designation and fast track status may provide additional market protection. Generic companies should carefully evaluate these exclusivity periods before planning market entry.
Market Entry Assessment & Recommendations
Based on the comprehensive analysis of Entresto’s patent landscape and market position:
- Patent Barrier Assessment: Entresto has multiple layers of patent protection extending to 2037, with the strongest barriers being the formulation and new use patents in the Orange Book. While some early formulation patents will expire by 2025, later patents on specific formulations, dosing regimens, and manufacturing processes extend protection significantly.
- Generic Entry Strategy:
- The 18 ANDAs submitted in 2019 with first approval in May 2024 indicate generic interest, but careful patent navigation is required.
- Consider focusing on developing around the expired or soon-to-expire patents while designing non-infringing alternatives to the later-expiring patents.
- Evaluate Paragraph IV certification strategy for challenging certain patents while respecting others.
- Innovator Defense Strategy:
- Continue developing new formulations and indications to extend product lifecycle.
- The new capsule formulations approved in 2024 represent an effective lifecycle management strategy.
- Focus on pediatric indications and additional heart failure subtypes to expand market reach.
- Market Differentiation:
- For generics: Highlight bioequivalence and cost advantages when entering the market.
- For innovator: Emphasize the proven clinical outcomes from PARADIGM-HF and subsequent studies.
- Consider value-based contracts with payers based on hospitalization reduction metrics.
- Regulatory Considerations:
- Monitor ongoing clinical trials for potential label expansions.
- Consider the impact of recent FDA guidance on complex drug products.
- Evaluate international markets where patent protection may differ from the US.
The overall assessment suggests that while generic entry for certain formulations may begin in 2025 after key patent expirations, comprehensive market entry will be challenging until the mid-2030s due to Novartis’s robust patent strategy covering multiple aspects of the product. Companies should consider focused strategies targeting specific formulations or indications where patent barriers are lower.
For more scientific and detailed information of Entresto, try PatSnap Eureka Pharma CI Explorer.
Only takes 4 steps to unlock the detailed result 👇

