
In thermodynamics, two fundamental concepts play a critical role in understanding the energy dynamics of a system: enthalpy and entropy. While they both describe different aspects of a system’s behavior, they are interrelated and essential for analyzing energy transfer, chemical reactions, and the spontaneity of processes. This article will dive deep into the key differences, formulas, applications, and examples of enthalpy vs. entropy, shedding light on their roles in both theoretical and practical thermodynamics.
What is Enthalpy?
Enthalpy (H) refers to the total heat content of a system. It is used to measure the energy required for a process occurring at constant pressure. This thermodynamic quantity is important in processes like heating, cooling, chemical reactions, and phase transitions. Enthalpy is typically measured in joules (J), and its change, ΔH\Delta HΔH, determines whether heat is absorbed or released in a system.
Formula for Enthalpy: ![]() Where:
Where:
- H is the enthalpy.
- U is the internal energy.
- P is the pressure.
- V is the volume.
What is Entropy?
Entropy (S) is a measure of the disorder or randomness within a system. It reflects the number of possible microstates that a system can exist in, with higher entropy indicating greater disorder. Entropy is crucial for determining the spontaneity of a process, as it increases in irreversible processes and dictates the direction of natural processes. It’s measured in joules per kelvin (J/K).
Formula for Entropy: ![]() Where:
Where:
- ΔS is the change in entropy.
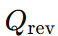 is the heat exchanged in a reversible process.
is the heat exchanged in a reversible process.- T is the temperature.
Key Differences Between Enthalpy and Entropy
Enthalpy and entropy serve different purposes in thermodynamics, and understanding their distinctions is essential for grasping the dynamics of energy in systems. Here’s a detailed comparison of the two concepts:
| Property | Enthalpy | Entropy |
|---|---|---|
| Definition | Total heat content of a system, related to energy transfer. | Measure of disorder or randomness in a system. |
| Formula | ||
| Units | Joules (J) | Joules per Kelvin (J/K) |
| Role in Thermodynamics | Describes energy required for heat transfer at constant pressure. | Describes the degree of disorder and irreversibility. |
| Behavior | Increases with heat added to the system at constant pressure. | Increases in spontaneous processes, reflecting disorder. |
| Key Application Areas | Chemical reactions, phase changes, and heat transfer. | Spontaneous processes, entropy increase in isolated systems. |
| Related Law | First Law of Thermodynamics (conservation of energy). | Second Law of Thermodynamics (entropy tends to increase). |
| Change in State | Enthalpy change (ΔH\Delta HΔH) used to determine heat released or absorbed. | Entropy change (ΔS\Delta SΔS) determines whether a process is spontaneous. |
| Sign of Change | Negative for exothermic reactions (heat released), positive for endothermic reactions (heat absorbed). | Positive for irreversible processes, negative for reversible ones. |
| Relation to Work | Associated with the work done by the system in constant pressure processes. | Indicates irreversibility, limiting efficiency in energy conversion. |
Real-World Applications of Enthalpy vs. Entropy
Enthalpy in Chemical Reactions
In chemical reactions, the change in enthalpy, ΔH, determines whether the reaction is exothermic (releases heat) or endothermic (absorbs heat). For instance, the combustion of fuels like methane is exothermic, releasing heat. This understanding is crucial for designing energy-efficient processes in industries like power generation.
Entropy in Spontaneous Processes
The second law of thermodynamics states that the entropy of an isolated system always increases over time. For example, the mixing of two gases in a container leads to an increase in entropy as the molecules disperse and occupy more available microstates. This principle is crucial in the design of engines, refrigerators, and other systems where the direction of natural processes is important.
💡 Looking to understand the real-world applications of enthalpy and entropy? Eureka Technical Q&A provides detailed explanations of how these thermodynamic concepts are applied in fields like energy production, chemical engineering, and climate science, helping you grasp their significance in practical scenarios.
Case Studies and Examples
- Heat Transfer in Refrigerators: In refrigerators, the enthalpy change helps measure the heat absorbed from the inside of the fridge, while the entropy change determines the efficiency of the refrigeration cycle. The efficiency of the system depends on minimizing the increase in entropy, which is why engineers prefer refrigerants with low entropy changes.
- The Burning of Fuels: Consider the burning of wood. The enthalpy change reflects the heat you release during combustion, while the entropy change shows how much disorder you introduce into the system as the fuel transitions from solid to gas.
Conclusion
Both enthalpy and entropy are integral concepts in thermodynamics, each serving its distinct role. While enthalpy is primarily concerned with the heat content and energy transfer at constant pressure, entropy deals with the randomness and disorder of a system. Together, they provide a comprehensive understanding of energy transformations, heat flow, and the spontaneity of natural processes. Mastery of these concepts is essential for fields such as engineering, chemistry, and environmental science.
FAQs
1. How do enthalpy and entropy relate to spontaneous processes?
While enthalpy deals with heat exchange, entropy is directly related to the spontaneity of a process. A process is spontaneous when the total entropy of the system and surroundings increases, as described by the second law of thermodynamics.
2. Can entropy ever decrease in a system?
Yes, entropy can decrease locally in a system, but the total entropy of an isolated system must always increase, in accordance with the second law of thermodynamics.
To get detailed scientific explanations of Enthalpy vs. Entropy, try Patsnap Eureka.


