
Electrostatic force, also known as Coulomb’s force, is the force of attraction or repulsion between two electrically charged particles. This fundamental force governs many physical interactions in nature and plays a crucial role in modern technology. In this article, we will explore Coulomb’s force, how it differs from gravitational force, its real-world applications, and commonly asked questions.
Principles of Coulomb force
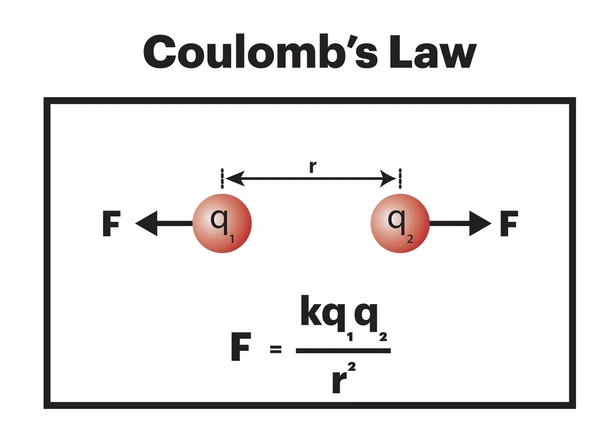
The Coulomb’s force between two point charges is described by Coulomb’s law, formulated by French physicist Charles-Augustin de Coulomb in 1785. The law states that the magnitude of the electrostatic force (F) between two charges is directly proportional to the product of the magnitudes of the charges (q₁ and q₂) and inversely proportional to the square of the distance (r) between them:
![]()
F = Electrostatic force (Newtons)
q₁, q₂ = Magnitudes of the two charges (Coulombs)
r = Distance between the charges (meters)
kₑ = Coulomb’s constant ≈ ![]()
If the charges have the same sign (both positive or both negative), they repel each other.
If the charges have opposite signs (one positive, one negative), they attract each other.
Factors Affecting Electrostatic Force
- Magnitude of Charges: Larger charges result in a stronger force.
- Distance Between Charges: Increasing the distance between charges decreases the force exponentially, as it follows an inverse-square law.
- Medium Between Charges: The presence of different materials between charges can affect the force due to variations in permittivity.
Electrostatic Force vs. Gravitational Force
Both electrostatic and gravitational forces obey the inverse-square law, meaning their strength decreases as the square of the distance increases. However, they have fundamental differences, as shown in the table below:
| Feature | Electrostatic Force | Gravitational Force |
|---|---|---|
| Nature of Force | Can be attractive or repulsive | Always attractive |
| Cause | Acts between charged particles | Acts between masses |
| Governing Law | Coulomb’s Law: Depends on charge magnitudes and distance | Newton’s Law of Universal Gravitation: Depends on mass and distance |
| Equation | (F = k_e \frac{ | q₁ q₂ |
| Constant Used | Coulomb’s constant ke=8.9875×109k_e = 8.9875 × 10^9ke=8.9875×109 N·m²/C² | Gravitational constant G=6.674×10−11G = 6.674 × 10^{-11}G=6.674×10−11 N·m²/kg² |
| Strength | Much stronger than gravitational force | Much weaker compared to electrostatic force |
| Example | Electrons repelling or attracting in an atom | Planets orbiting the Sun due to gravity |
Key Differences:
- Electrostatic force is much stronger than gravitational force, but it acts only on charged objects.
- Gravity is a universal force that acts between all objects with mass, but it is much weaker than electrostatic force.
Applications of Electrostatic Force
💡 Curious about the applications of electrostatic forces? Eureka Technical Q&A provides expert insights into how electrostatics are utilized in technologies like laser printers, inkjet printers, and electrostatic painting, helping you understand their role in modern devices and industrial processes.
Electrostatic forces are integral to numerous applications:
- Photocopiers and Laser Printers: These devices use electrostatic charges to attract toner particles onto paper, creating images and text.
- Electrostatic Precipitators: Used in industrial settings to remove particulate matter from exhaust gases, improving air quality.
- Capacitors: Electronic components that store energy using electrostatic fields between conductive plates.

FAQs
- How does Coulomb’s force differ from gravitational force?
While both forces follow an inverse-square law concerning distance, electrostatic force can be either attractive or repulsive, depending on the charges involved. In contrast, gravitational force is always attractive and acts between masses. - Can electrostatic forces act over long distances?
Electrostatic forces can act over considerable distances; however, their strength diminishes rapidly as the distance between charges increases, following the inverse-square law. - How are electrostatic forces utilized in everyday life?
Electrostatic forces are employed in various everyday applications, such as in photocopiers, where they help transfer toner to paper, and in air purifiers, where they assist in removing dust particles from the air. - Why don’t we notice Coulomb’s force in daily life?
Objects around us are mostly electrically neutral. Charged objects, such as static electricity from clothes, reveal the effects of Coulomb’s force.
Conclusion
Coulomb’s force is a fundamental interaction that shapes our world at the atomic level and is essential in modern technology. Compared to gravitational force, Coulomb’s force is stronger, can be repulsive or attractive, and acts between charged particles. From photocopiers to lightning, electrostatic forces play a role in many natural and engineered processes.
Understanding electrostatic interactions helps in electronics, environmental technology, and material sciences, making them a crucial part of physics and engineering.
To get detailed scientific explanations of Electrostatic Force, try Patsnap Eureka.


