Overview
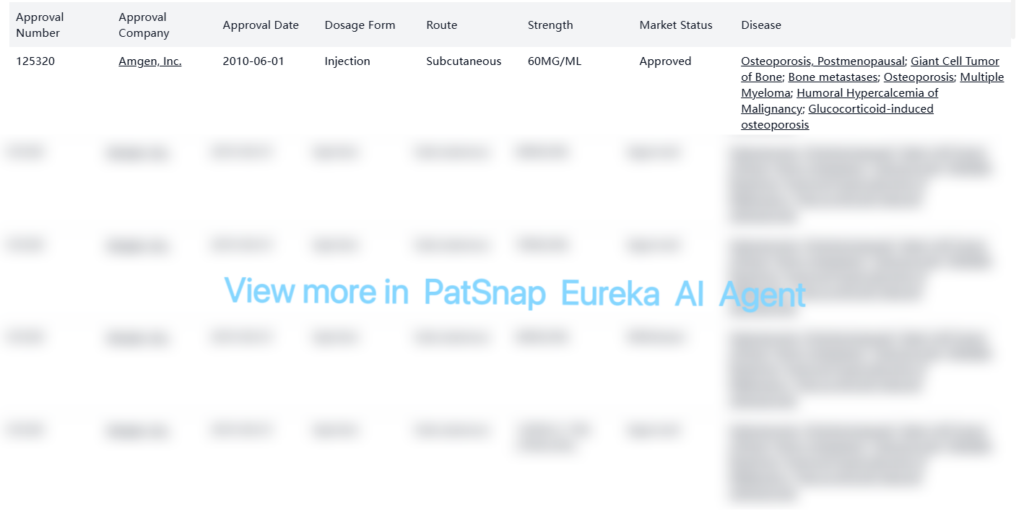
Denosumab is an approved biological drug in the U.S. market, with a single reference product developed by Amgen, Inc. As a RANKL inhibitor, Denosumab has received regulatory approval for multiple indications, including osteoporosis, giant cell tumor of bone, and bone metastases. Its clinical significance and commercial potential are underscored by the emergence of 32 biosimilars, such as the Denosumab biosimilar developed by Biocon, reflecting a highly competitive landscape.
Drug Information
Denosumab received its first U.S. FDA approval on June 1, 2010, and is available in various formulations for indications such as:
Biosimilars: There are 32 biosimilars of Denosumab currently identified, including notable entries such as Biocon’s version.
Special Regulatory Designations
Denosumab has received orphan drug designations for specific indications in the United States:
| Organization | Indication | Special Review | Country | Approval Date |
|---|---|---|---|---|
| Amgen, Inc. | Giant Cell Tumors | Orphan Drug | United States | 2010-12-20 |
| Amgen, Inc. | Hypercalcemia | Orphan Drug | United States | 2013-09-11 |
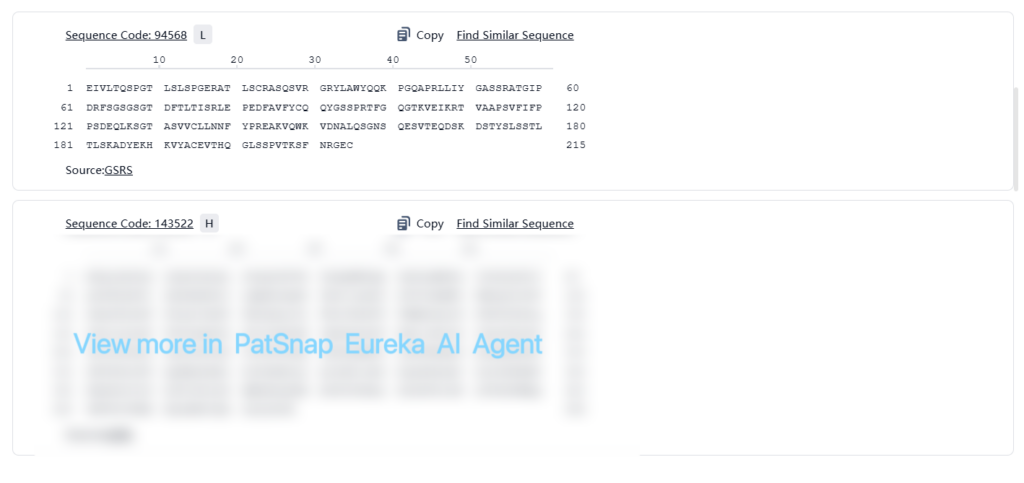
Structural Information
Registration Patent Barrier Analysis
Denosumab is protected by numerous patents listed in the FDA Purple Book. Below is a summary of key registration patents:
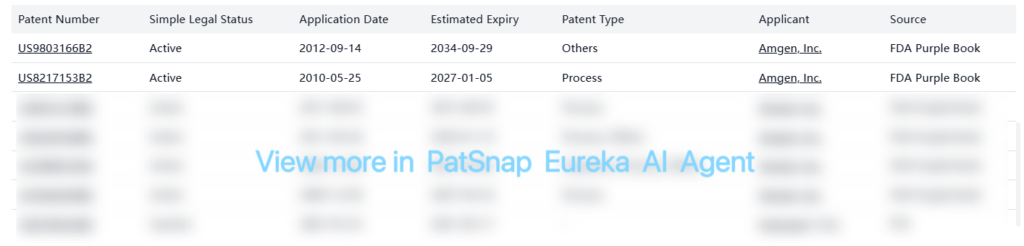
The patents cover various aspects of Denosumab, including manufacturing processes, formulations, and other related technologies. Many of these patents extend protection until the 2030s, with some key patents expiring as late as 2034. The PTE (Patent Term Extension) patent US6740522B2 has already expired as of September 17, 2021.
Other Patent Barrier Analysis
Amgen also holds numerous other patents related to Denosumab that may provide additional protection:
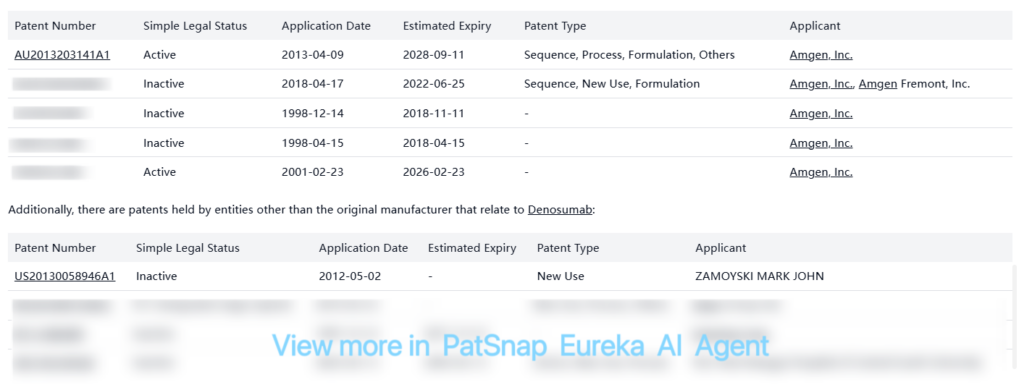
These patents cover various aspects including new uses, processes, and formulations related to Denosumab. Some are still active, while others have expired or been designated as inactive.
Clinical Study Insights
Based on the FDA Label Clinical Insight, Denosumab has been extensively studied in multiple clinical trials:
- Pharmacodynamic and Mechanism of Action Studies:
- Denosumab binds to RANKL, inhibiting its interaction with RANK on osteoclasts
- A single 60 mg dose reduces bone resorption marker serum C-telopeptide (CTX) by ~85% within three days
- Effects are reversible at the end of each dosing interval
- Immunogenicity Experiments:
- Less than 1% of patients developed binding antibodies after up to 5 years of treatment
- No patients developed neutralizing antibodies
- No clinically significant impact on pharmacokinetics, safety, or efficacy
- Clinical Trials in Osteoporosis:
- Studies in postmenopausal women assessed effects on bone turnover and BMD
- Common adverse reactions included back pain, pain in extremity, and musculoskeletal pain
- A randomized, double-blind, placebo-controlled study in men with osteoporosis (n=240) evaluated safety endpoints
- A 3-year multinational trial assessed effects in men with nonmetastatic prostate cancer on ADT (n=1468)
Infringement Cases
No infringement cases related to Denosumab were found in our database.
Policy and Regulatory Risk Warning
After a comprehensive search, Denosumab has data exclusivity protection in the United States as a biological product. Under the Biologics Price Competition and Innovation Act (BPCIA), original biologics receive 12 years of exclusivity from the date of first approval. Since Denosumab was first approved in 2010, this exclusivity period has expired, allowing for biosimilar applications and approvals.
Market Entry Assessment & Recommendations
Based on the analysis of Denosumab’s patent landscape and regulatory status in the US:
- Patent Protection: Denosumab still maintains significant patent protection through multiple patents that extend into the 2030s, with key manufacturing process patents expiring as late as 2034. This creates substantial barriers for generic entry.
- Biosimilar Competition: Despite patent protection, the presence of 32 biosimilars indicates strong interest in this market. Companies developing biosimilars should:
- Focus on patent workaround strategies for manufacturing processes
- Consider challenging the validity of key patents or seeking licensing agreements
- Prepare for potential patent litigation with Amgen
- Market Differentiation Strategies:
- For the originator (Amgen): Develop extended-release formulations, combination therapies, or new indications to maintain market share
- For biosimilar developers: Emphasize cost advantages and comparable efficacy/safety profiles
- Consider device innovations for easier administration to differentiate products
- Regulatory Strategy:
- The expiration of biological exclusivity allows for biosimilar applications
- Companies should engage with FDA early to understand specific requirements for demonstrating biosimilarity
- Consider the potential for interchangeability designation to gain market advantage
- Market Access Considerations:
- Develop robust payer engagement strategies focusing on cost-effectiveness
- Consider patient assistance programs to improve access and adherence
- Target specific patient subpopulations where Denosumab has shown particular benefits
- Long-term Planning:
- Monitor patent litigation developments closely
- Prepare for potential authorized biosimilars from Amgen as patent expiry approaches
- Consider strategic partnerships to strengthen market position and distribution capabilities
The market for Denosumab represents a significant opportunity given its multiple indications and established efficacy profile, but entrants must carefully navigate the complex patent landscape and develop clear differentiation strategies to succeed.
For more scientific insights, patent analysis, and biosimilar strategy tools, try PatSnap Eureka AI Agent— your intelligent partner for life science innovation.