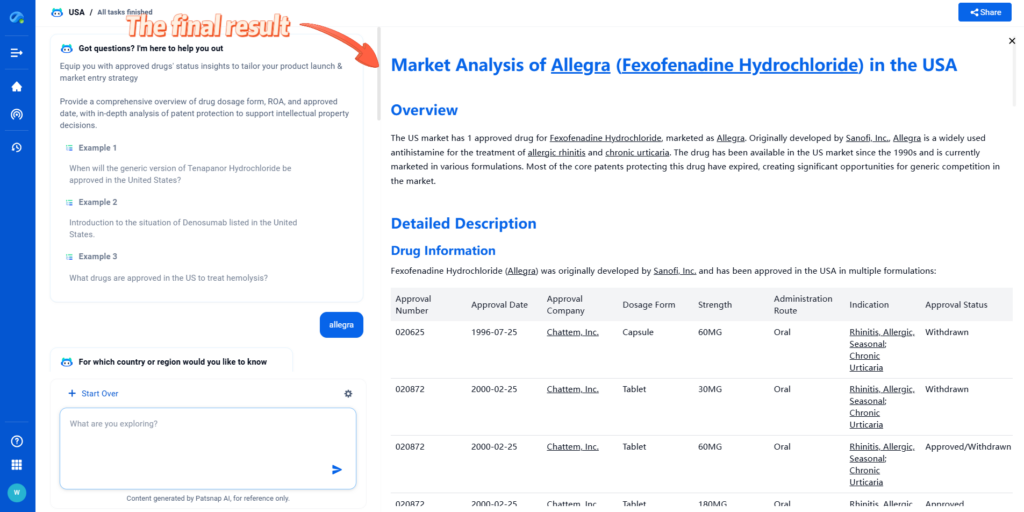
Overview
The US market has 1 approved drug for Fexofenadine Hydrochloride, marketed as Allegra. Originally developed by Sanofi, Inc., Allegra is a widely used antihistamine for the treatment of allergic rhinitis and chronic urticaria. The drug has been available in the US market since the 1990s and is currently marketed in various formulations. Most of the core patents protecting this drug have expired, creating significant opportunities for generic competition in the market.
Detailed Description
Drug Information
Fexofenadine Hydrochloride (Allegra) was originally developed by Sanofi, Inc. and has been approved in the USA in multiple formulations:

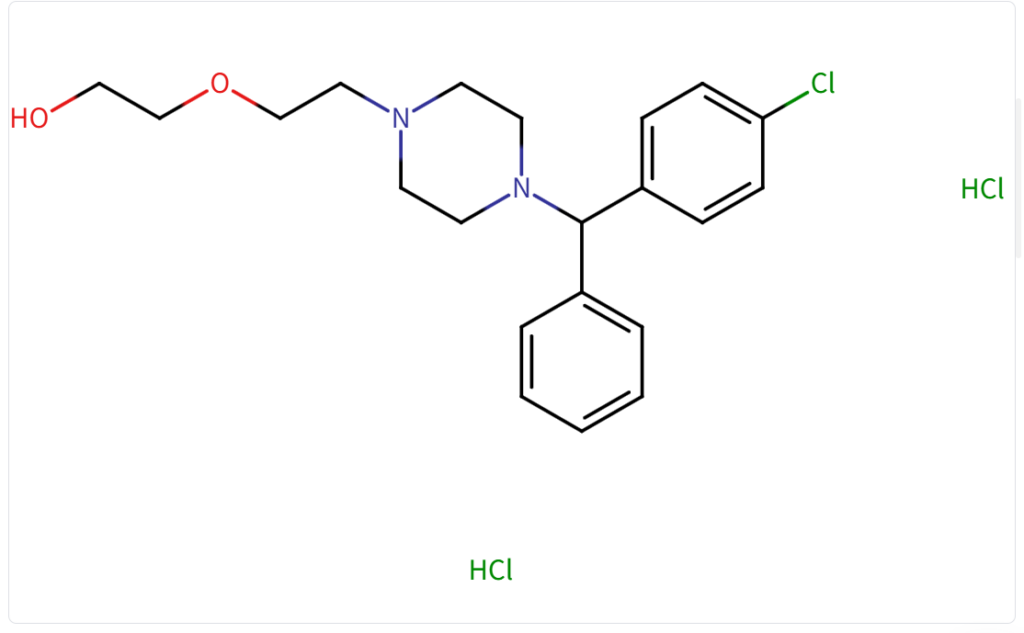
Structure
Patent Statement Information
There are patent statements for Allegra in the US:
| Trade Name | Submission Date |
|---|---|
| Allegra | Pre-MMA |
Additionally, for the suspension formulation:
| Trade Name | Submission Date | ANDAs Submit Number | 180-day Status | Decision Posting Date | First Applicant Approval Date | First Commercial Marketing Date |
|---|---|---|---|---|---|---|
| Allegra | 2010-01-25 | 1 | Eligible | 2019-11-19 | 2014-11-18 | 2014-12-22 |
Registration Patent Barrier Analysis
The FDA Orange Book lists multiple patents for Fexofenadine Hydrochloride, all of which have expired:
PTE patent:
| Patent Number | Simple Legal Status | Application Date | Estimated Expiry | Patent Type | Applicant | Source |
|---|---|---|---|---|---|---|
| US4254129A | Inactive | 1979-04-10 | 2001-02-17 | New Use, Product Derivative, Product Compound, Formulation | Merrell Dow Pharmaceuticals, Inc. | PTE |
Other Patent Barrier Analysis
There are numerous other patents from various companies related to Fexofenadine Hydrochloride, including:

Key patents to note include:
- US8933097B2 (expires 2030) – An active formulation patent from Chattem, Inc. for a suspension formulation
- CN102070490B (expires 2029) – An active patent from Zhejiang Huahai for a manufacturing intermediate
- CN105287554A (expires 2034) – An active patent from Shenyang Pharmaceutical University for a drug combination
Clinical Results
Based on FDA label information, Fexofenadine Hydrochloride is approved for the temporary relief of allergy symptoms. The clinical data indicates that:
- The drug is effective for allergic rhinitis and chronic urticaria
- It is generally administered in 12-hour dosing intervals
- There are specific dosage recommendations for adults, children over 6 years of age, and patients with kidney disease
- Warnings exist regarding potential drug interactions with antacids and fruit juices
The FDA label does not provide detailed information about specific clinical trials that led to approval, but the dosage guidelines and safety information are based on comprehensive clinical research including randomized controlled trials, pharmacokinetic studies, and safety assessments.
Infringement Cases
No specific patent infringement cases related to Fexofenadine Hydrochloride were found in the available data.
Policy and Regulatory Risk Warning
After a comprehensive search, no market exclusivity or data protection period was identified for Fexofenadine Hydrochloride in the USA. Most of the core patents have expired, with only a few formulation and combination patents remaining active.
Market Entry Assessment & Recommendations
Based on the patent and market analysis, the following recommendations can be made:
- Generic Market Entry: Most core patents for Fexofenadine Hydrochloride have expired, making it a viable target for generic manufacturers. However, specific formulations, particularly the suspension formulation (US8933097B2, valid until 2030), remain protected.
- Formulation Innovation: Companies interested in this market should consider developing novel formulations or delivery systems to bypass existing patents. Potential areas include:
- Extended-release formulations
- Alternative delivery systems (e.g., nasal sprays, transdermal applications)
- Taste-masked pediatric formulations
- Combination Products: Consider developing combination products with other active ingredients to address multiple symptoms or conditions, keeping in mind the active patent CN105287554A for compound anti-cold medicine combinations.
- Market Differentiation: With multiple generics likely available, market differentiation strategies should focus on:
- Improved patient convenience (e.g., once-daily dosing)
- Enhanced taste profiles for pediatric formulations
- Patient support programs
- Cost advantages through manufacturing efficiencies
- Regulatory Strategy: For generic manufacturers, focus on formulations where patents have expired. For innovator companies, consider:
- Developing improved formulations with clinical benefits
- Exploring new indications beyond allergic rhinitis and urticaria
- Pursuing OTC switch opportunities in markets where the drug is still prescription-only
- Geographic Expansion: Consider markets outside the US where patent protection may differ or where market penetration of Fexofenadine products is lower.
- Supply Chain Optimization: Develop efficient API manufacturing processes to reduce costs, particularly for generic competition.
In conclusion, while Fexofenadine Hydrochloride faces significant generic competition in its basic form, opportunities exist for companies that can innovate on formulation, indication expansion, or cost leadership strategies.
For more detailed information of Fexofenadine Hydrochloride, try PatSnap Eureka Pharma CI Explorer.