
Introduction
Aldehydes and ketones are two important classes of organic compounds commonly studied in chemistry. Both contain a carbonyl group (a carbon double-bonded to oxygen), but they differ in structure, properties, and applications. In the comparison of aldehyde vs. ketone, understanding their key differences and similarities is essential for grasping their roles in chemical reactions, industrial processes, and biological systems. This article will break down their distinctions, shared characteristics, and real-world uses to provide a clear and detailed comparison.
What Are Aldehydes?
Aldehydes are organic compounds with a carbonyl group (C=O) attached to a terminal carbon atom. Their general formula, RCH=O, includes a hydrogen bonded to the carbonyl carbon. In formaldehyde (H₂CO), the simplest aldehyde, the “R” group is replaced by hydrogen. This unique structure makes aldehydes highly reactive and ideal for various chemical reactions.
Key Properties and Characteristics
High Chemical Reactivity
The carbonyl group in aldehydes is highly reactive, enabling them to easily undergo oxidation, reduction, and other transformations. Many reactions also involve the adjacent carbon atom, adding to their versatility.
Physical States
The physical state of aldehydes depends on their carbon chain length. Lower aldehydes (C1 to C8) are usually gases or liquids with strong, pungent odors. In contrast, higher aldehydes (above C8) are solids with pleasant scents, making them popular in perfumes and flavoring products.
Toxicity Concerns
Aldehydes can cause irritation to the eyes, skin, and respiratory tract with short-term exposure. Long-term exposure to formaldehyde or acetaldehyde increases the risk of serious conditions like cancer and cardiovascular diseases.
What Are Ketones?
Ketones: Structure, Properties, and Natural Sources
Ketones are organic compounds featuring a carbonyl group (C=O) bonded to two carbon-containing substituents (R-C(=O)-R’). This structure gives ketones their unique chemical properties and versatility. Naturally, ketones are found in plants, where they serve as key components in essential oils. They are also synthesized industrially for applications in fragrances, food additives, and healthcare products.
Key Properties and Characteristics
Natural Production
Ketones like β-ionone, known for its woody, violet-like aroma, form through the oxidative cleavage of carotenoids. These compounds play a major role in creating rose ketones, widely used in the food and fragrance industries.
Sustainable Synthesis Challenges
Traditional methods for producing natural ketones rely on chemical synthesis or extraction from plants. While effective, these methods are costly and unsustainable. Biosynthetic processes, such as lipoxygenase or peroxidase-mediated carotenoid cleavage, offer more eco-friendly alternatives but still require optimization to manage metabolite flux.
Fossil Material Ketones
Ketones found in fossil materials like crude oil and coal tar belong to various classes, including n-alkanones and aromatic ketones. Some contain additional oxygen or nitrogen atoms. Mass spectrometry analysis of these compounds helps scientists understand fossil material composition and stability.
Aldehyde vs. Ketone: Key Differences
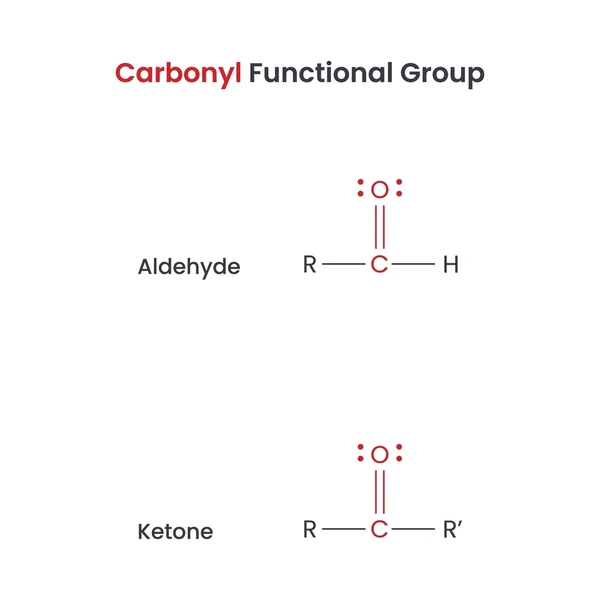
Aldehydes and ketones share the carbonyl group (C=O), but their structure, reactivity, and properties differ significantly. Understanding these differences is essential for identifying their unique roles in chemistry.
Location of the Carbonyl Group
In aldehydes, the carbonyl group appears at the end of the carbon chain, bonded to at least one hydrogen atom. In ketones, the carbonyl group lies within the carbon chain and connects to two carbon atoms or side chains.
Structure and Bonding
Both aldehydes and ketones feature sp² hybridized carbon atoms, arranged in a trigonal planar geometry. However, aldehydes include at least one hydrogen atom bonded to the carbonyl carbon, while ketones do not.
Oxidation Behavior
Aldehydes oxidize easily into carboxylic acids, making them more reactive. Ketones, on the other hand, resist oxidation and remain more stable under typical conditions.
Reactivity
Both compounds undergo nucleophilic addition reactions, but aldehydes react faster because of the hydrogen atom’s influence on the carbonyl carbon. Ketones can also participate in keto-enol tautomerism, forming a reactive enol intermediate.
Solubility
Ketones are generally more water-soluble than alcohols and carboxylic acids of similar weight. Their carbonyl group allows for effective hydrogen bonding with water molecules.
Volatility
Ketones tend to be more volatile than alcohols and carboxylic acids of the same molecular weight. This is because they lack strong intermolecular attractions, unlike their counterparts.
Naming Conventions
Aldehydes take the suffix “-al,” such as propanal for a three-carbon chain with an aldehyde group. Ketones replace the suffix with “-one,” as seen in propanone for a similar chain with a ketone group.
Aldehyde vs. Ketone: Common Reactions
Aldehydes and ketones share a variety of important chemical reactions due to their carbonyl group. These reactions make them versatile in organic synthesis and industrial applications.
Nucleophilic Addition Reactions
Both aldehydes and ketones undergo nucleophilic addition, where a nucleophile attacks the carbonyl carbon. For example, reactions with hydroxylamine produce oximes, while hydrazine forms hydrazones.
Oxidation and Reduction
Aldehydes oxidize easily into carboxylic acids, while ketones are typically reduced to secondary alcohols. These reactions involve electron transfer at the carbonyl group, changing the compound’s functional state.
Aldol Condensation
In aldol condensation, aldehydes or ketones react with other carbonyl compounds in the presence of a base. This forms β-hydroxy carbonyl compounds, essential in synthesizing complex organic molecules.
Cyanation Reactions
Catalytic asymmetric cyanation produces optically active cyanohydrins from aldehydes and ketones. These cyanohydrins serve as valuable building blocks in pharmaceuticals and natural product synthesis.
Enolization and Keto-Enol Tautomerism
Both compounds can undergo enolization, forming an equilibrium between keto and enol forms. This tautomerism is critical for their reactivity in various chemical reactions.
Alpha Substitution Reactions
At the alpha position to the carbonyl group, aldehydes and ketones can undergo substitution. Enolates formed during this process act as nucleophiles, allowing for the introduction of new groups.
Reactions with Carbon Dioxide
Aldehydes can couple with carbon dioxide to form valuable compounds. Catalysts often drive these reactions, making them useful for producing specialty chemicals.
Uses of Aldehydes and Ketones in Everyday Life
Solvents
Aldehydes and ketones are widely used as solvents in the production of lacquers, paints, varnishes, and even explosives. Acetone, for instance, dissolves a wide range of substances, making it a popular choice.
Production of Specialty Chemicals
Aldehydes play a key role in creating polymers, resins, dyes, plasticizers, and perfumes. They are also essential in producing flavorings, pharmaceuticals, and disinfectants for various industries.
Fuel Additives
Aldehydes, such as esters, are critical in biodiesel production. Fatty acid methyl or ethyl esters serve as eco-friendly alternatives to conventional fuels.
Consumer Products
Fragrances and Flavoring Agents
Low molecular weight esters are valued for their pleasant, volatile scents, making them ideal for perfumes and flavoring agents.
Cosmetics and Pharmaceuticals
Fatty alcohols derived from aldehydes act as emulsifiers, emollients, and thickeners in cosmetics. Both aldehydes and ketones are also used in manufacturing essential pharmaceuticals.
Textile Processing
Ketones contribute to textile processing by aiding in dye production and finishing applications, ensuring durability and vibrant colors.
Environmental and Health Considerations
Occupational Exposure
Exposure to aldehydes and ketones can be harmful through inhalation or skin absorption, potentially impacting the liver, kidneys, and nervous system.
Genotoxicity Risks
Some aldehydes can damage DNA, raising concerns about their presence in foods and drug products. Strict international guidelines help control and minimize these risks.
FAQ
1. Can both aldehydes and ketones be oxidized?
Aldehydes can be easily oxidized to carboxylic acids. Ketones are more resistant to oxidation and usually require strong oxidizing agents to undergo further oxidation.
2. Are aldehydes and ketones soluble in water?
Lower molecular weight aldehydes and ketones are soluble in water due to their ability to form hydrogen bonds with water molecules. As the carbon chain length increases, their solubility in water decreases.
3. How do the boiling points of aldehydes and ketones compare?
Both have higher boiling points than hydrocarbons of similar molecular weight due to the polar nature of the carbonyl group. However, their boiling points are generally lower than those of alcohols because they cannot form hydrogen bonds with themselves.
To get detailed scientific explanations of Aldehyde vs. Ketone, try Patsnap Eureka.

