Overview
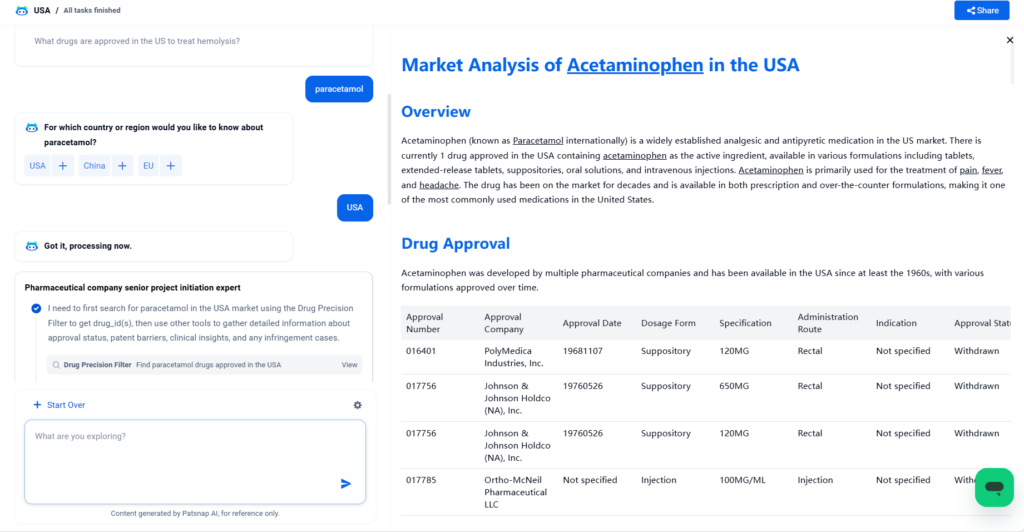
Acetaminophen (known as Paracetamol internationally) is a widely established analgesic and antipyretic medication in the US market. There is currently 1 drug approved in the USA containing acetaminophen as the active ingredient, available in various formulations including tablets, extended-release tablets, suppositories, oral solutions, and intravenous injections. Acetaminophen is primarily used for the treatment of pain, fever, and headache. The drug has been on the market for decades and is available in both prescription and over-the-counter formulations, making it one of the most commonly used medications in the United States.
Drug Approval
Acetaminophen was developed by multiple pharmaceutical companies and has been available in the USA since at least the 1960s, with various formulations approved over time.
Patent Statements

Patent Barrier
Registration Patent Analysis
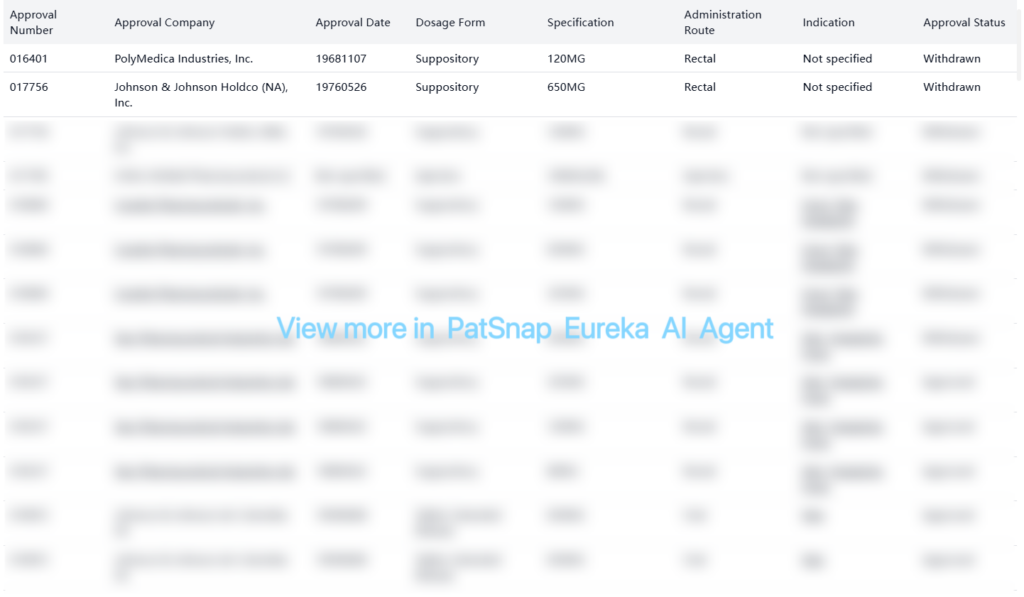
The FDA Orange Book lists several patents for acetaminophen formulations, with varying expiration dates. Some key patents include:
Other Patent Barrier Analysis
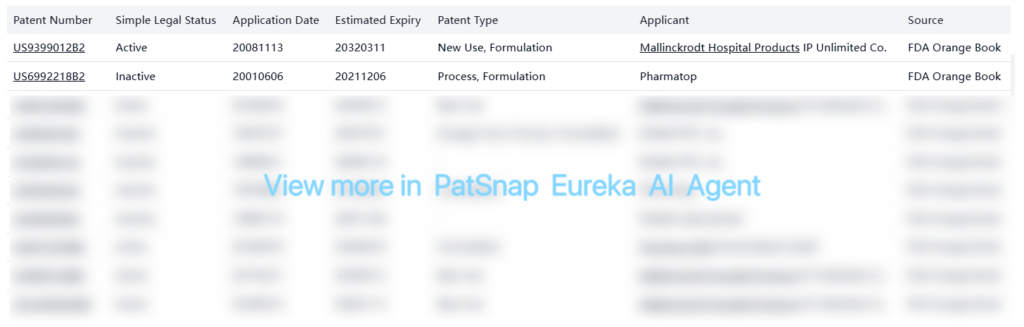
There are numerous non-registration patents related to acetaminophen formulations, manufacturing processes, and combinations with other drugs. Most of these patents are inactive or expired, but several remain active:
| Patent Number | Simple Legal Status | Application Date | Estimated Expiry | Patent Type | Applicant |
|---|---|---|---|---|---|
| US11197830B2 | Active | 20190227 | 20390227 | Dosage Form, New Use, Formulation | AFT Pharmaceuticals Ltd. |
| US8715952B2 | Active | 20120709 | 20290417 | Diagnostic, Analysis and Assay | Sekisui Diagnostics LLC |
| CN115844840A | Active | 20221207 | 20421207 | Process, Formulation | Guangdong Medi-World Pharmaceutical Co., Ltd. |
| CN65a92126 | Active | 20100716 | 20300716 | Process, Formulation | Yueyang Xinhuada Pharmaceutical Co Ltd. |
| CN102861040B | Active | 20121011 | 20321011 | Process, Formulation | Guangdong Medi-World Pharmaceutical Co., Ltd. |
The patent landscape shows that while many basic patents for acetaminophen have expired, there are still active patents covering specific formulations, delivery methods, and combination therapies. These patents are primarily held by companies like Mallinckrodt Hospital Products, Fresenius Kabi, and various Chinese pharmaceutical manufacturers.
Clinical Results
1. Adult Clinical Studies
A. Acute Pain Studies
- Study 1 (Postoperative Pain Following Total Hip or Knee Replacement):
- Design: Two randomized, double-blind, placebo-controlled clinical trials.
- Population: 101 adult patients suffering from moderate to severe postoperative pain after total hip or knee replacement surgery.
- Interventions: Patients were administered repeated doses of acetaminophen injection (1,000 mg) every 6 hours for 24 hours versus placebo.
- Outcomes: The study demonstrated that acetaminophen injection was statistically superior to placebo in reducing pain intensity over 24 hours. An additional observation was a reduction in opioid consumption, although the clinical benefit regarding opioid sparing was not explicitly demonstrated.
- Study 2 (Postoperative Pain Following Abdominal Laparoscopic Surgery):
- Design: A randomized, double-blind, placebo-controlled clinical trial.
- Population: 244 adult patients with moderate to severe postoperative pain after abdominal laparoscopic surgery.
- Interventions: Two dosing regimens were explored—1,000 mg every 6 hours and 650 mg every 4 hours for 24 hours—compared with placebo.
- Outcomes: Both dosing regimens with acetaminophen injection provided a statistically significant greater reduction in pain intensity over the 24-hour period compared to placebo.
B. Fever Study
- Design: A randomized, double-blind, placebo-controlled clinical trial using an endotoxin-induced fever model.
- Population: 60 healthy adult males.
- Interventions: A single dose of acetaminophen injection (1,000 mg) was administered, and the patients were observed over 6 hours.
- Outcomes: The study demonstrated a statistically significant antipyretic effect of acetaminophen injection compared to placebo, as reflected by a decrease in mean body temperature over time.
2. Pediatric Clinical Studies
- Study Designs:
- The pediatric population was involved in three active-controlled trials and three open-label safety and pharmacokinetic trials.
- Population Breakdown:
- A total of 483 pediatric patients participated, including neonates, infants, children, and adolescents.
- Interventions and Regimens:
- Dosing schedules included administrations up to 15 mg/kg of acetaminophen injection every 4 hours, every 6 hours, or every 8 hours.
- Additional Details:
- The maximum exposure duration varied slightly by age group—up to 7.7 days for neonates, 6.4 days for infants, 6.8 days for children, and 7.1 days for adolescents.
- Outcomes:
- In these studies, the focus was on both demonstrating the efficacy in alleviating acute pain and fever, as well as assessing safety and pharmacokinetic profiles in the pediatric population.
3. Safety and Adverse Reaction Studies
- Adverse Reaction Monitoring in Adults:
- A total of 1,020 adult patients were evaluated in the clinical trials to capture treatment-emergent adverse reactions.
- Observations:
- Common adverse reactions (with a frequency greater than placebo) included nausea, vomiting, headache, insomnia, anemia, and other events such as infusion site pain and edema.
- Serious Reactions Highlighted:
- Serious adverse reactions including hepatic injury, serious skin reactions, and hypersensitivity responses were discussed in the labeling and monitored during these studies.
- Adverse Reaction Monitoring in Pediatrics:
- In pediatric trials, a range of adverse events were similarly recorded.
- Observations:
- The most common adverse events in pediatric patients included nausea, vomiting, constipation, and pruritus.
- Additional events such as diarrhea, pyrexia, and injection site pain were observed at a lower incidence (<5%).
Policy and Regulatory Risk Warning
After a comprehensive search, acetaminophen does not currently have market exclusivity or data protection periods in the United States. Most of the basic patents for acetaminophen have expired, allowing for widespread generic production. However, specific formulations and delivery methods may still be covered by active patents.
The FDA has implemented various regulatory actions regarding acetaminophen over the years:
- In 2011, the FDA limited the strength of prescription acetaminophen-containing products to 325 mg per tablet/capsule to reduce the risk of liver injury.
- The FDA requires all prescription acetaminophen products to include a Boxed Warning highlighting the potential for severe liver injury.
- Over-the-counter acetaminophen products must include warnings about liver damage and alcohol use.
These regulatory measures do not prevent generic competition but do impose specific labeling and formulation requirements that must be followed by all manufacturers.
Market Entry Assessment & Recommendations
Market Assessment
- Patent Landscape: Most basic acetaminophen patents have expired, but specific formulations (particularly intravenous formulations) remain under patent protection until the late 2020s and early 2030s.
- Market Competition: The acetaminophen market is highly competitive with numerous generic manufacturers for oral formulations. The intravenous market has fewer competitors due to active patent protection.
- Regulatory Environment: While there are no exclusivity barriers, there are strict FDA requirements regarding labeling, dosage strength, and safety warnings.
Recommendations for Market Entry
- Formulation Innovation:
- Develop novel formulations with improved bioavailability, reduced hepatotoxicity, or extended-release properties
- Focus on pediatric-specific formulations which may qualify for additional regulatory incentives
- Consider combination products with complementary analgesics or other active ingredients
- Differentiation Strategies:
- Invest in taste-masking technologies for oral liquid formulations to improve palatability, particularly for pediatric populations
- Develop unique delivery systems (e.g., transdermal patches, orally disintegrating tablets) that may obtain new patent protection
- Create value-added formulations with improved stability or convenience features
- Regulatory Strategy:
- For generic products, focus on ANDA submissions for formulations with expired patents
- For innovative formulations, pursue 505(b)(2) pathway to leverage existing safety and efficacy data while adding novel features
- Consider pursuing specific indications or patient populations not currently addressed by existing products
- Manufacturing Optimization:
- Implement cost-effective manufacturing processes to compete effectively in the highly competitive generic market
- Develop high-quality, consistent formulations that meet or exceed current Good Manufacturing Practice (cGMP) requirements
- Consider vertical integration to control raw material costs and ensure supply chain reliability
- Market Access Strategies:
- For prescription products, focus on hospital formulary inclusion and physician education
- For OTC products, invest in brand differentiation and consumer marketing
- Consider private label partnerships with major retailers to gain market share
- Risk Mitigation:
- Implement robust pharmacovigilance systems to monitor for adverse events, particularly hepatotoxicity
- Develop clear patient education materials about appropriate dosing and risks
- Consider product liability insurance and legal consultation regarding warning labels
The acetaminophen market in the USA remains attractive despite being mature, with opportunities for innovation in formulation, delivery systems, and specific patient populations. Companies entering this market should focus on differentiation, cost-effectiveness, and regulatory compliance to succeed in this competitive landscape.
For more detailed information of Acetaminophen, try PatSnap Eureka AI Agent.